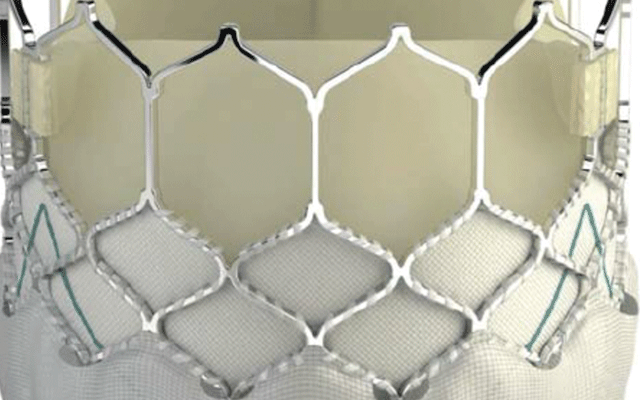
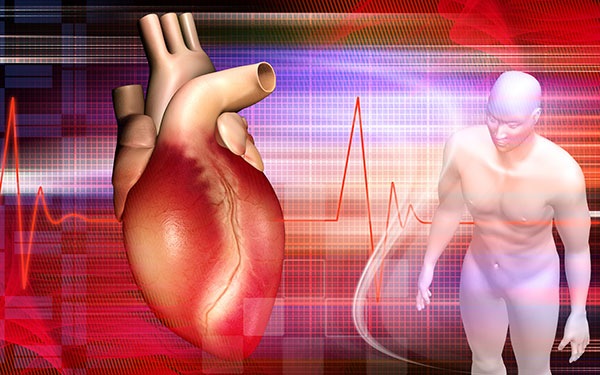
A new study, published in Circulation: Cardiovascular Interventions, shows t...
This content is only for readers outside of the US as it discusses a device ...
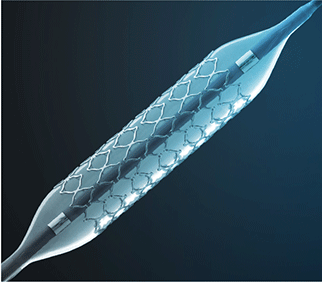
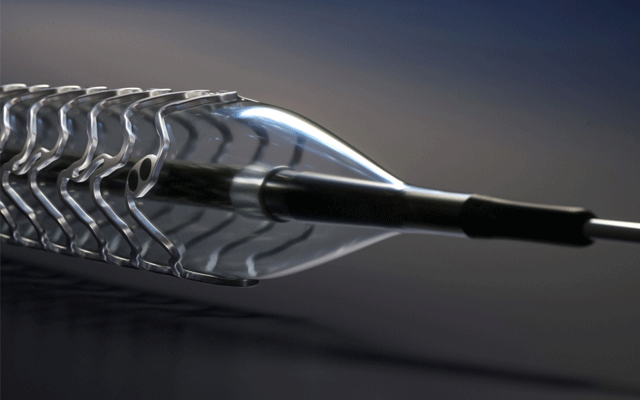
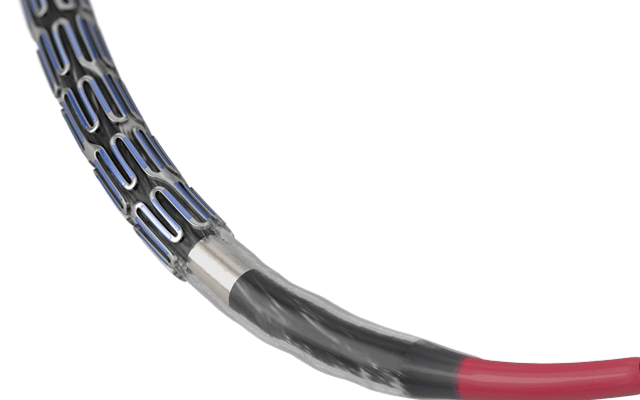
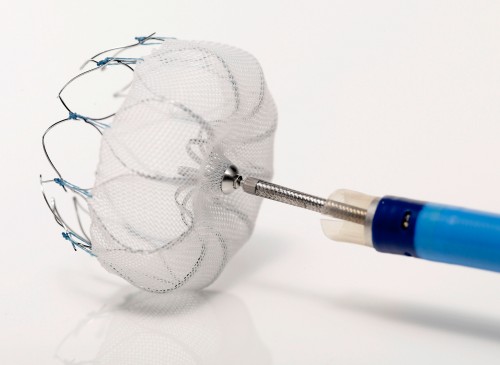
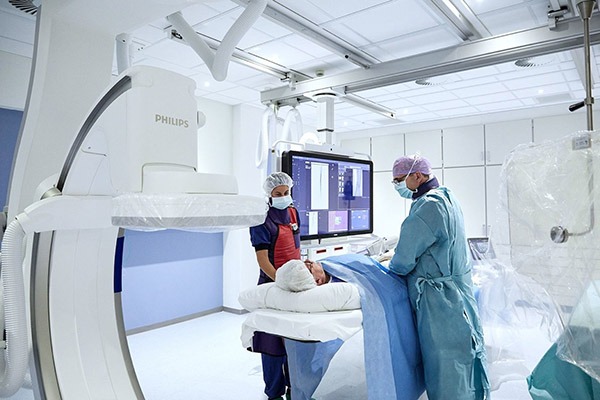
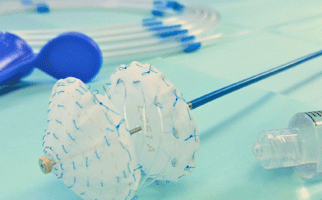
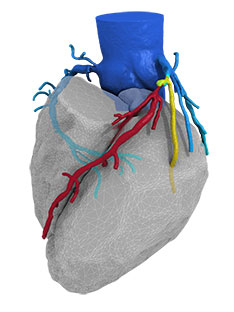
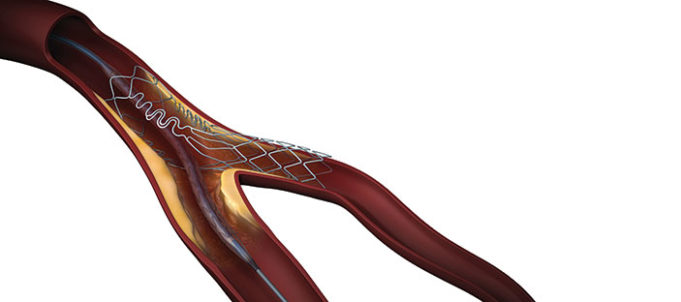
Results from the SAFE MANTA IDE clinical trial—the first pivotal trial for a...
The annual census of UK consultants and higher speciality trainees—Focus on ...
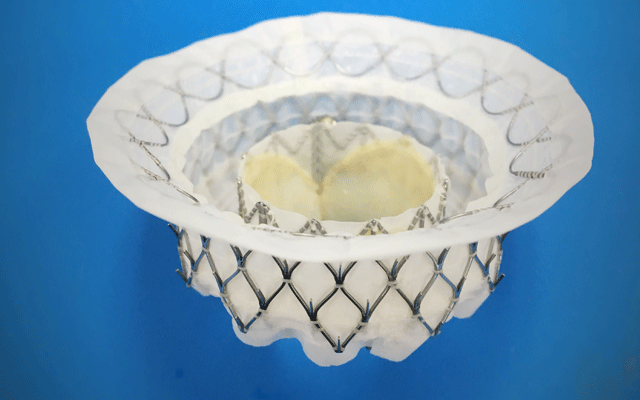
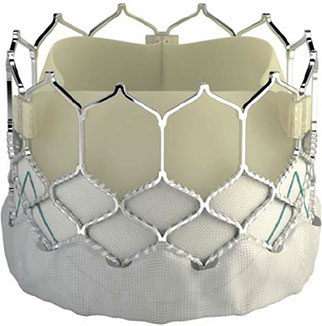
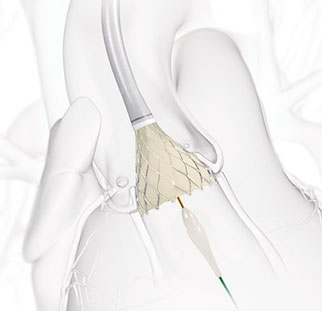
JenaValve Technology has announced the initiation of patient enrolment and i...
According to a study published in LGBT Health, bisexual men have a higher ri...
Steven Farmer (Center for Healthcare Innovation and Policy Research, George ...
A pre-specified analysis of the TROPICAl-ACS study indicates that de-escalat...
Keystone Heart announced the launch of phase II of the REFLECT trial, which ...
Ron Waksman (MedStar Heart & Vascular Institute, Washington, DC, USA) is...
Bay Labs has received US FDA 510(k) clearance for its EchoMD AutoEF software...
Caladrius has revealed that the US FDA has granted regenerative medicine advance...
A new study, SYNTAX III REVOLUTION, has found heart teams using computed tom...
A new study indicates that a delayed approach in patients with transient ST-...
According to Anja Stundl (Department of Medicine II, Heart Center Bonn, Univ...
In this PCR video, David E Kandzari (Department of Interventional Cardiology, Pi...
New data from ADAPT-DES, published in JACC: Cardiovascular Interventions, in...
Hermann Reichenspurner (Departments of Cardiovascular Surgery and General an...
Corindus Vascular Robotics has received Pharmaceutical and Medical Device Ag...
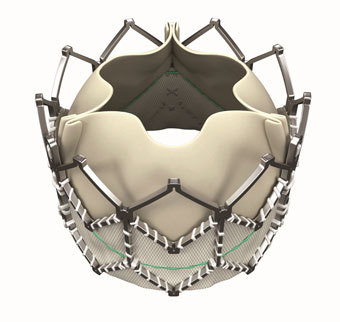
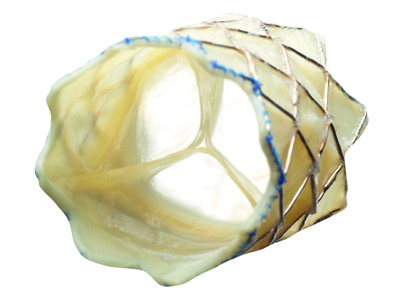
The NOTION trial (Nordic aortic valve intervention) was the first comparison...
Countries in the European Union have long been the first to receive new innovati...
Thirty-day data presented at EuroPCR (22 May—25 May, Paris, France) show con...
The American College of Cardiology (ACC), Heart Rhythm Society (HRS), North ...
Five-year data from FAME (Fractional Flow Reserve vs. Angioplasty for Multiv...
According to a study published in BMJ Open, just over half of patients think...
For our May 2018 edition (Issue 49), we report on the key studies from the 2018 ...
For our May 2018 edition (Issue 49), we report on the key studies from the 2018 ...
Data presented at the British Cardiovascular Society Conference (4–6 June, Manch...
Biomerics has bought FutureMatrix Interventional, which specialises in the desig...
The US FDA has classified Medtronic’s recent voluntary urgent field action r...
New data from the ORBITA trial indicates percutaneous coronary intervention ...
Compared with first-generation devices, new-generation transcatheter aortic ...
At EuroPCR (22 May–25 May, Paris, France), the 12-month results of the ANGIO...
The clinical results from the OxAMI-PiCSO study—published in EuroInterventio...
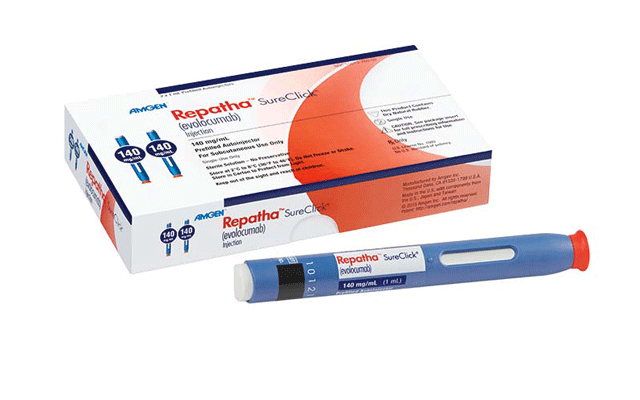
The European Commission (EC) has authorised a new indication in the evolocum...
Amaranth Medical provided an update on its sirolimus-eluting bioresorbable scaff...
CeloNova BioSciences has announced one-year clinical trial results from the ...
Two-year outcome data from the BIO-RESORT randomised controlled trial, which...
Investigators unveiled clinical data from the independently run Onyx 1-Month...
Alvimedica unveiled the details of the Diab8 study—the first diabetic drug-eluti...
This content is for distribution within the UK only.
This content is ...
Abbott has announced it has received approval from the US FDA for its Xience...
Data presented during EuroPCR (22 May – 25 May, Paris, France) provide furth...
After two studies indicated that renal denervation lowers blood pressure bot...
New data from the PACIFIC study presented at EuroPCR (22 May – 25 May, Paris, Fr...
Intravascular Lithotripsy (Shockwave Medical) is now available, on the European ...
A press release reports that the first patient to receive the GATE tricuspid val...
Ancora Heart has announced the expansion of its US feasibility study to eval...
Boston Scientific Corporation has revealed its schedule of key presentations...
CoreMedic has announced the start of the first-in-man Chagall study to evaluate ...
Medtronic has announced the initiation of a clinical study in the US to asse...
Pi-Cardia has recently started its first-in-human study with its Leaflex Perform...
A new study shows that patients who undergo percutaneous coronary interventi...
Erik W Holy (Heart Center, Segeberger Kliniken, Bad Segeberg, Germany) and o...
Cardiac Dimensions has appointed Matthew Stark as vice president of clinical and...
Abbott has announced that Japan’s Ministry of Health Labour and Welfare (MHLW) h...
According to a study published in Circulation, among more than 230,000 cardi...
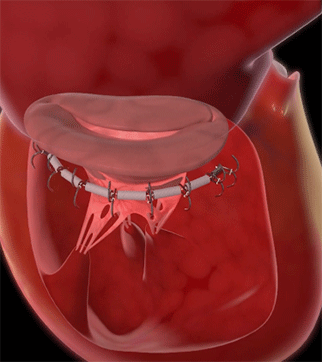
Edwards Lifesciences has received the CE mark for the Cardioband tricuspid v...
David A Cox, (Brookwood Baptist Health, San Antonio, USA; Cardiovascular Associa...
A new study presented at the 2018 Society for Cardiovascular Angiography and...
Speaking at the 2018 CRT meeting (3–6 March, Washington, DC), Victor Alfonso...
Patients who require non-emergent, uncomplicated target lesion revascularisa...
One-year outcome data for transcatheter mitral valve implantation (TMVI), in...
Terumo has received the CE mark for its Ultimaster Tansei drug-eluting stent...
Root cause analysis of radial occlusion points to vessel trauma. An ultrasound s...
A review of the trends in the use and propensity-matched analysis of in-hosp...
Roberto Canessa is one of 16 survivors from Uruguayan Air Force Flight 571, ...
The COBRA REDUCE trial is exploring the safety and efficacy of prescribing d...
Intravascular lithotripsy, or IVL (Shockwave), is a novel procedure that is ...
According to a press release, Boston Scientific has, along with several othe...
A press release reports that HeartStitch transapical access and closure device p...
Medtronic has announced that Laura Mauri—interventional cardiologist and clinica...
New data show that, after a myocardial infarction, significantly fewer women...
Supporting the results of previous studies, a new study indicates that percu...
A new study indicates that just over a third of patients with cardiovascular...
Abbott has initiated the landmark GUIDE-HF clinical trial using the CardioME...
Abiomed has received US FDA premarket approval (PMA) for its Impella CP hear...
Following the results of the Gore REDUCE clinical study, which found that cl...
Medtronic has announced US FDA approval to begin an investigational device e...
A press release reports that successful patient enrolment in the CE mark stu...
Nine and 12-month data for Orsiro—presented by Yang Yue Jin (Fu-Wai Hospital...
Edwards Lifesciences has announced that enrolment is complete in the compute...
Colibri Heart Valve has announced that the first two patients have been succ...
Acurate neo
Boston Scientific has announced the UK Court of Appeal dism...
The European Hospital in Rome has become the first site in Italy to participate ...
Justin Davies (Imperial College London, London, UK) has always been interest...
According to a new study, the higher rate of all-cause mortality after percu...
Data from the YOUNG-MI registry indicate that 10% of young myocardial infarc...
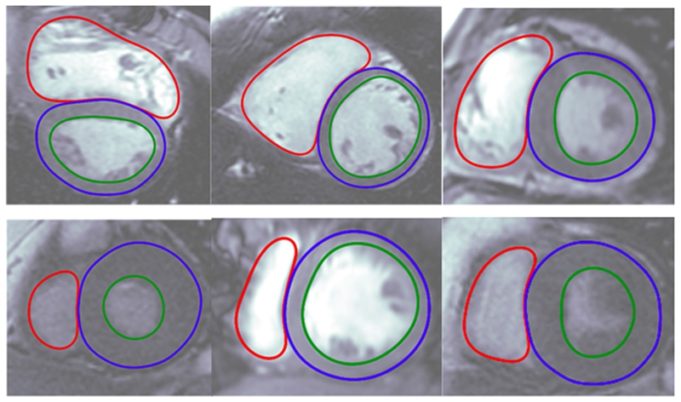
Transcatheter aortic valve implantation (TAVI) has progressed from being a p...
Data from the SMART-DATE study indicate that acute coronary syndrome patient...
A new study—presented at the American College of Cardiology (ACC) Scientific Ses...
Data indicate that patients who undergo TAVI with the Sentinel Cerebral Protecti...
Biotronik has announced that patient enrolment for the BIOSTEMI study has be...
Economic data from the DEFINE FLAIR clinical trial indicate that using instantan...
New data from the CoreValve US Pivotal Extreme Risk Study and the real-world...
The US FDA has approved the Masters HP 15mm rotatable mechanical heart valve...
Umesh Khot (Cleveland Clinic, Cleveland, USA) and colleagues found that implemen...
The ODYSSEY OUTCOMES trial met its primary endpoint, demonstrating that high-ris...
C Michael Valentine is the new president of the American College of Cardiolo...
Cardiovascular Systems has announced that the US FDA has granted 510(k) clea...
HeartFlow has entered into a licensing and technology transfer agreement with Ce...
Data from the FITT-STEMI (Feedback intervention and treatment times in ST-se...
Registry data for younger patients—under 75 years—undergoing transcatheter a...
Karl-Heinz Ladwig (Technical University of Munich and Helmholtz Zentrum Münc...
According to a press release, Reva Medical has received the CE mark for its ...
According to a study presented at the American College of Cardiology’s Cardiovas...
Designed specifically for small vessels, the Resolute Onyx 2mm drug-eluting ...
In this edition of Cardiovascular News (issue 48, US edition), we report on the ...
In this edition of Cardiovascular News (issue 48), we report on the top stories ...
The Food and Drug Administration (FDA) has today issued the final rule on “h...
Edwards Lifesciences has received the CE mark for its self-expanding Centera tra...
Felix Weise (Cardioangiologisches Centrum Bethanien, Frankfurt, Germany) and...
New data from the EXCEL trial, which found that percutaneous coronary interv...
Since it received FDA clearance in June 2017, the Sentinel cerebral protecti...
Abiomed has received an expanded US FDA premarket approval for the Impella 2...
The first patient has been enrolled in a clinical trial evaluating 28 days o...
LivaNova has entered into an agreement to acquire TandemLife, a privately held c...
Somahlution has announced that Golden Jubilee National Hospital and Victoria Bla...
According to an American College of Cardiology (ACC) press release, hundreds...
A new subanalysis of the phase III PEGASUS-TIMI 54 trial indicates that tica...
BioVentrix has announced a significant reimbursement achievement. InEk, the ...
BTG reported that the OPTALYSE PE one-year trial results, presented at ISET (3–5...
Thomas Munzel (Department of Internal Medicine, University Medical Center Mainz,...
At the 2018 Joint EuroCMR/SCMR meeting (31 January–3 February, Barcelona, Spain)...
HeartFlow has revealed that seven new commercial payers issued positive medi...
Data from the FRALITY-AVR (Frality aortic valve replacement) study suggest t...
Jeffrey C Kwong (Institute for Clinical Evaluative Sciences, Toronto, Canada...
Robocath—a company that conceives and develops cardiovascular robotic system...
BioCardia has announced that the US FDA has approved an investigational device e...
According to a press release from Tryton Medical, the first patient has been...
Corindus Vascular Robotics will be co-sponsoring courses at multiple leading...
Surmodics has received US FDA 510(k) clearance for its Telemark .014inch coronar...
Uchenna R Ofoma (Geisinger Health System, Danville, USA) and others report i...
Boston Scientific has closed an investment and entered into an acquisition o...
According to a Biotronik press release, the Japanese Ministry of Health has ...
The European Society of Cardiology (ESC) has released a new video, see below, th...
An all-comers study of patients undergoing percutaneous coronary interventio...
Patients who undergo transfemoral transcatheter aortic valve implantation (T...
Nearly a quarter of patients with chronic ischaemic cardiovascula...
Cordis and Medinol have announced that the first US commercial cases using t...
Corindus Vascular Robotics has announced that it is working with Mayo Clinic...
The Christ Hospital, Lindner Research Center in Cincinnati, USA, has enrolled th...
The results of PRECLUDE, an analysis of data from the ongoing SWEDEHEART qua...
Endotronix has announced successful first-in-human implantation of the Corde...
Acarix started the initiation of a multicentre trial of its handheld CADScor...
Essential Medical has announced the completion of the 60-day follow up for i...
A meta-analysis, published in The American Journal of Medicine, provides fur...
Miracor Medical Systems and Miracor Medical have announced the closing of € ...
A meta-analysis—published in Circulation Cardiovascular Interventions—indica...
Andrew Goldsweig (Division of Cardiology, University of Nebraska Medical Cen...
According to a press release, a first-in-man feasibility study focusing on a...
The Science Museum (London, UK) has unveiled a special exhibit to commemorat...
HeartFlow has announced that the Health Care Service Corporation (HCSC)—that...
Janssen has submitted a supplemental new drug application to the US FDA for ...
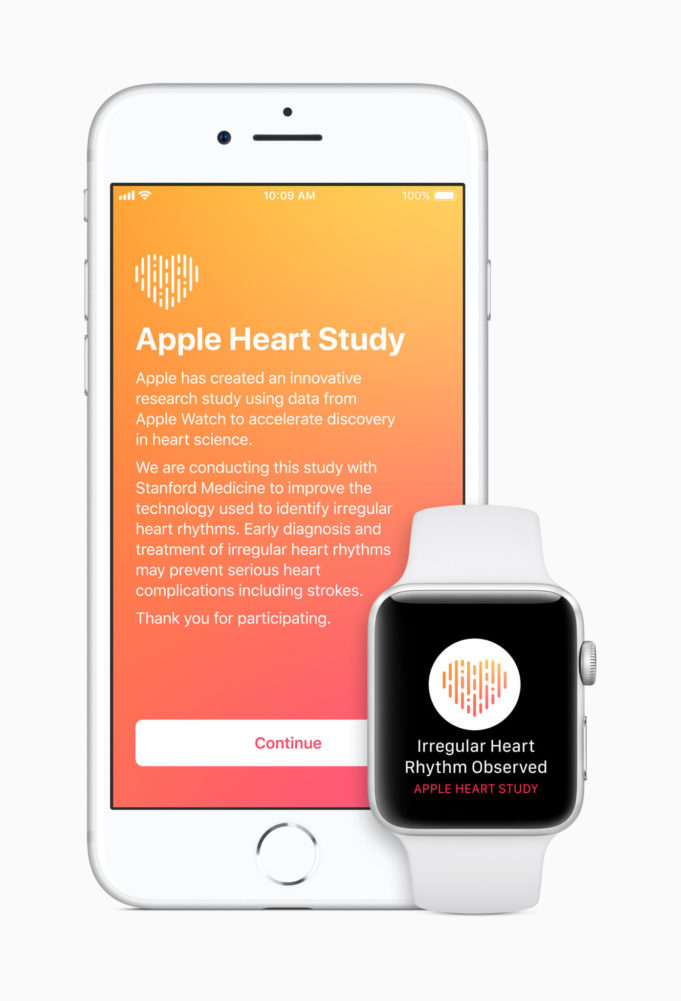
Apple has launched the Apple Heart Study app, a first-of-its-kind research study...
The American Heart Association (AHA) has named attorney M Lewis Kinard as ex...
Philips has acquired VitalHealth, a provider of cloud-based population health ma...
Edwards Lifesciences Corporation today announced it has acquired Harpoon Medical...
4C Medical Technologies has won the Best Technology Parade Presentation award—at...
The iSTEMI study, published in JACC: Cardiovascular Interventions, suggests ...
Fan Chung (National Heart & Lung Institute, Imperial College London, UK)...
According to the interim results of the first-ever multi-country online surv...
Avinainder Singh (Brigham and Women’s Hospital, Harvard Medical School, Bost...
Data from the regional emergency cardiovascular (RESCUe) network indicate th...
CorInnova has been awarded second prize in the “2017 InnoSTARS” life science...
The HREVS (Hybrid coronary revascularisation vs. standard) trial found that ...
Following ORBITA, which indicated percutaneous coronary intervention (PCI) d...
The US FDA has approved evolocumab (Repatha, Amgen) for the prevention of my...
Data from the ABSORB IV study indicate that device thrombosis with the biore...
We are witnessing a new era in which artificial intelligence is being applied to...
A report—modified from US global health recommendations from the National Academ...
Cordis and Medinol have announced that the US FDA has approval the Elunir dr...
NeuroproteXeon has announced the publication of a second finding from a randomis...
The first human implantation of the VenusA Plus retrievable valve system was...
The Society for Cardiovascular Angiography and Interventions (SCAI) has laun...
The past few years has witnessed increasing clinician engagement with social...
Boston Scientific has announced a delay to the previously communicated timel...
Joseph Ladapo (David Geffen School of Medicine, University of California, Los An...
Data from the MITRAL (Mitral Implantation of Transcatheter valves) study ind...
Boston Scientific has announced the final five-year outcomes data from the P...
Francesco Maisano (University Heart Centre, University Hospital Zurich Zuric...
Data from PRESERVE (Prevention of serious adverse events following angiograp...
Primary results from the REDUCE LAP-HF trial indicate that a novel interatri...
Creavo Medical Technologies (Creavo) has won two awards at the Ins...
The ORBITA (Objective randomised blinded investigation with optimal medical ...
A study published in the Journal of the American College of Cardiology indicates...
Corvia Medical has enrolled the first patients in its global multicentre trial (...
Stentys has announced the precommercialisation of its Serpentis stent. According...
Philips has announced the results of a comprehensive, independent, two-year ...
Data published, as a research letter, in the Journal of the American College...
Neovasc has received approval of the US FDA to initiate the COSIRA-II IDE clinic...
ORBITA (Objective randomised blinded investigation with optimal medical ther...
Advice on percutaneous coronary intervention (PCI) strategies for patients w...
Abbott has announced that Japan's Ministry of Health, Labour and Welfare (MH...
HeartFlow has announced that the Centers for Medicare & Medicaid Service...
Elixir Medical unveiled for the first time its novel stent technology—Dynamx...
Data presented during the 2017 Transcatheter Cardiovascular Therapeutics (TC...
Acist has announced the global launch of its RXi Mini system—the next-generation...
The primary results from the DAPT-STEMI trial suggest that 12-month dual ant...
In this video, hear how key recommendations from the 2017 ESC/EACTS Guidelines f...
The Fantom bioresorbable scaffold (Reva Medical) shows continued positive cl...
Early experiences with two transcatheter mitral valve implantation (TMVI) de...
Olivier Varenne (Hôpital Cochin, Assistance Publique—Hôpitaux De Paris, Pari...
David Cohen (Saint Luke’s Mid America Heart Institute, Kansas City, USA) has...
New clinical research, presented today at the 2017 Transcatheter Cardiovascula...
New results from the HARMONEE Japan/US registration trial, which were presente...
The CE mark has been awarded to a new-generation of Abbott Vascular's everol...
New data from the ongoing CADLAD (Coronary artery disease learning and algorithm...
Highlights:
SPYRAL HTN-OFF MED provides "proof of principle" that renal den...
Highlights:
SPYRAL HTN-OFF MED provides "proof of principle" that renal den...
Bay Labs, a medical technology company developing an artificial intelligence in ...
A team at the Technical University of Munich (TUM) has determined that in the fi...
Nitiloop has received FDA clearance for its new Nova Cross Extreme and Nova Cros...
The US FDA has approved the expansion of CeloNova’s ongoing clinical trial of it...
David Montaigne (University of Lille, France) and others report in The Lance...
A new study indicates that long-term exposure to elevated blood pressure is ...
Data from the TRANSLATE-ACS (Treatment with adenosine diphosphate receptor i...
In Scientific Reports, a Nature publication, Julien Bec (Department of Biome...
Boston Scientific has announced key data will be presented at next week’s Tr...
Claret Medical has announced that it has closed on a series C financing of U...
The first patient in the APOLLO trial has undergone transcatheter mitral val...
Micro Interventional has announced that the second successful implantation of it...
Everolimus-Eluting Platinum Chromium Coronary Stent System
Drug-eluting s...
Data presented at the 8th Emirates Cardiac Society congress (19–21 October, ...
Thomas Haldis (Sanford Health, Fargo, USA) and others report in EuroInterven...
Reva Medical has announced the commercial expansion of its bioresorbable sca...
Elixir Medical has revealed details of its programme and activities during the 2...
The American College of Cardiology (ACC), Heart Failure Society of America (...
A new study indicates that coronary angiography, with or without percutaneou...
The US FDA has granted 510(k) clearance to Creavo Medical Technologies for i...
James W Hansen (Lahey Hospital and Medical Center, Burlington, USA) and othe...
Garen Wintemute (Violence Prevention Program, University of California Davis...
Bernard Prendergast (Department of Cardiology, St. Thomas' Hospital, London, UK)...
Vivasure Medical has announced the successful enrolment of the first patient...
The Geoffrey O. Hartzler Master Clinical Operator Award will be presented to Ale...
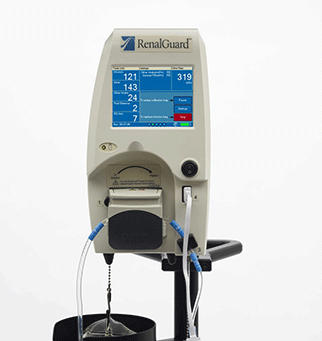
A first-in-man, feasibility study of the use of the RenalGuard system in pat...
Acarix AB has presented its CADScor system for non-invasive, non-radiation a...
A retrospective study conducting a safety assessment and compariso...
Pamela B Morris has been selected as the next vice chair of the American Col...
Biotronik has announced the start of enrolment in a coronary stent trial aiming ...
At CSI UCSF Congress, Achille Gaspardone (Hospital of Sant'Eugenio, Rome, Italy)...
Thomas Lüscher has been appointed director of education, research and develo...
Vascular Graft Solutions is hosting a symposium on the future of coronary ar...
Fahad Alqahtani (Division of Cardiology, West Virginia University Heart &...
BioVentrix has announced that the first patient has been enrolled in the US ...
Essential Medical has announced the completion of enrolment in the US pivotal in...
The first patient was treated in the CONTROL hypertension (HTN)-2 clinical study...
A study published in the Journal of the American College of Cardiology indic...
Stefan Verheye (Antwerp Cardiovascular Centere ZNA Middelheim, Interventional Ca...
Factors such as delayed diagnosis may contribute to the differences in women and...
The independent Data Safety Monitoring Board (DSMB) has found no significant...
The US Food and Drug Administration (FDA) has approved Medtronic’s HeartWare...
Gore has completed enrolment for the pivotal phase of its Gore ASSURED Clini...
New data from REPRISE III indicate that keeping the bottom of the frame at a...
The burden of cardiovascular mortality and morbidity is constantly increasing in...
It is now almost a decade since the first patient underwent catheter-based r...
The US Food and Drug Administration (FDA) has accepted TherOx’ Premarket Approva...
ReFlow Medical has received 510(k) clearance from the US Food and Drug Administr...
Spectranetics has recalled the Bridge occlusion catheter “due to the possibi...
Saranas has announced the appointment of Philippe Généreux as its chief medical ...
The American College of Cardiology (ACC) and the American Heart Association (AHA...
LivaNova has received FDA 510(k) clearance for the US market launch of its O...
iVascular SLU has received approval for selling its coronary and peripheral prod...
The president of the American College of Cardiology (ACC), Mary Norine Walsh...
A new post-market clinical study is to evaluate Medtronic’s CoreValve Evolut...
Abiomed has received FDA pre-market approval for its Impella RP heart pump. ...
Marco A Magalhaes (Department of Internal Medicine, MedStar Washington Hospi...
The results of CLOSE, Gore Reduce and the extended follow-up of RESPECT—all ...
Dragana Radovanovic (AMIS Plus Data Centre, Zurich, Switzerland) and colleag...
A new scanning device designed to aid cardiac ‘rule out’ is to be exhibited ...
Among patients who undergo the Fontan procedure, two-thirds of survivors req...
According to a new survey, the average age and income of cardiologists in the US...
As the field of structural heart interventions expands from the now-establis...
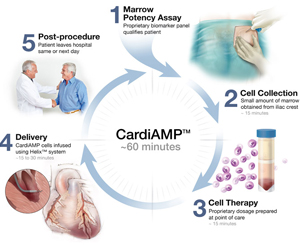
BioCardia has revealed the trial design for its pivotal Phase III CardiAMP Heart...
Carag has announced that it has completed CE marking for its Carag bioresorb...
Fysicon has announced that it has received 510(k) FDA clearance for its QMAP...
Jochen Wöhrle (Department of Internal Medicine II, Cardiology, University of...
There is a lack of sufficient data about the effect of racial differences on...
Anand Prasad (Department of Medicine, Division of Cardiology, University of ...
Evan Zahn (Guerin Family Congenital Heart Program, Cedars-Sinai Heart Institute,...
According to results from the FAST-MI programme, six-month mortality after S...
Patients. Our mission for life. Together, we can do even more than help pati...
The Cardiovascular Research Foundation (CRF) has release details of the 12 l...
Neovasc has provided an update on the study progress and clinical performanc...
Corindus Vascular Robotics has announced that the first patient has been enr...
Corey Adams (Health Science Center, St John’s, Canada) and others report in ...
Alvimedica has announced that the Court of Dusseldorf (Germany) has recognis...
Of 5,691 adverse events following transcatheter aortic valve implantation (T...
Data from DET02X-AMI (Determination of the role of oxygen in suspected acute...
Abbott has announced that it is to stop selling its first-generation bioreso...
Micro Interventional Devices has announced that its minimally invasive annulopla...
The recent DIVA (Drug-eluting stents vs. bare metal stents in saphenous vein...
Data from a nationwide observational cohort study indicate that coronary artery ...
In 2010, Harlan M Krumholz wrote a commentary in the Journal of the American...
The ProtEmbo cerebral protection system (Protembis) has been used, to comple...
The American College of Cardiology and several partnering societies—including th...
To mark angioplasty’s ruby anniversary, in this issue (47; September 2017), we b...
To mark angioplasty's ruby anniversary, in this issue (47; September 2017), we b...
C Michael Gibson (Cardiovascular Division, Department of Medicine, Beth Isra...
Mechanical reperfusion via primary percutaneous coronary intervention (PCI) ...
Despite a constant evolution in stent/scaffold design, stent failure is stil...
History was made on 16 September 1977 when Andreas Grüntzig performed world’s fi...
In this video from the European Society of Cardiology (ESC), Steen Dalby Kristen...
The FDA has approved the Full MagLev HeartMate 3 (Abbott) left ventricular a...
The European results of the first-in-human trial of Vascular Dynamics’ Mobiu...
The Gate catheter-guided tricuspid atrioventricular valved stent (NaviGate Cardi...
The COMPASS study, which was presented at the European Society of Cardiology...
Philips and HeartFlow have announced that they have entered into a collabora...
A new subanalysis from the phase III PEGASUS-TIMI 54 trial, which was presen...
Abbott has initiated a US pivotal clinical study evaluating the safety and e...
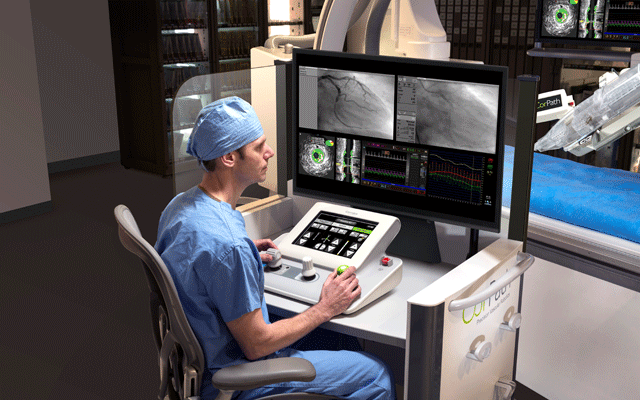
A robotic system for performing percutaneous coronary intervention (PCI) is ...
During a late-breaking science session at the European Society of Cardiology...
The SYNTAX II study has found that percutaneous coronary intervention (PCI) ...
Results from SPYRAL HTN-OFF MED indicate that renal denervation, compared wi...
Results from the BIOFLOW V study indicate that patients who underg...
The RE-DUAL PCI trial indicates that dual therapy with the non-vitamin K ant...
PG1ldGEgaHR0cC1lcXVpdj0icmVmcmVzaCIgY29udGVudD0iMDsgVVJMPSdodHRwOi8vd3d3LmJvc3Rv...
BioStable Science & Engineering has received US Food and Drug Administra...
New research has discovered a potential means to trigger damaged heart cells...
Physicians identified a majority of patients with advanced heart failure as at h...
Medtronic has announced a global randomised clinical trial that will evaluat...
Four Blue Cross Shield companies have issued positive medical policies for t...
Marijuana use is associated with a three-fold risk of death from hypertension, a...
LivaNova’s Perceval sutureless aortic heart valve has received approval from...
Children from socially and economically disadvantaged families and neighborhoods...
4C Medical Technologies has announced that its medical device therapy for mitral...
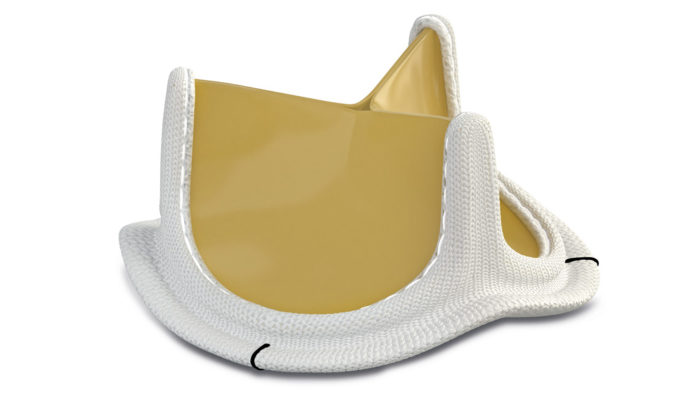
Medtronic has announced CE mark and FDA approval of its new Avalus pericardi...
SHS Gesellschaft für Beteiligungsmanagement is investing in CoreMedic, a start-u...
Scott Montgomery (Örebro University, Örebro, Sweden) and colleagues report i...
According to the US newspaper Star Tribune, Boston Scientific is to stop inv...
Keystone Heart has announced plans to initiate clinical trials for a new, ad...
Medtronic has received the CE mark for its CoreValve Evolut Pro valve for th...
Merit Medical will partner with internationally renowned interventional cardiolo...
National charity, Heart Research UK has granted funding for an innovative re...
Advances in materials and techniques over the past 40 years have led to a su...
A new study, XIENCE Short DAPT, will evaluate whether three months of dual a...
The American College of Cardiology (ACC) president Mary Norine Walsh has iss...
Roxwood Medical has entered into an exclusive agreement with Abbott for dist...
In a Viewpoint published in The Lancet, Pierre Carli (University Paris-Desca...
Using a new imaging technique that can diagnose cardiac sarcoidosis much more ac...
Millipede has announced the successful implantation of its newest 50mm Iris ...
The Committee for Medicinal Products for Human Use (CHMP) of the European Medici...
4C Medical has received approximately US$9 million in unsecured convertible prom...
Taisei Kobayashi (Corporal Michael J Crescenz, Veterans Affairs Medical Cent...
AstraZeneca has announced the UK availability of ticagrelor (Brilique) 90mg orod...
Cardiome Pharma has announced that the Therapeutic Products Directorate of H...
Philips has signed an agreement to acquire TomTec Imaging Systems GmbH, a provid...
Plant-based diets are recommended to reduce the risk of heart disease; however, ...
Kevin G Volpp (Center for Health Incentives & Behavioral Economics, Pere...
At this year’s EuroPCR (16–19 May, Paris, France), Philip MacCarthy writes, ...
Innovative Cardiovascular Solutions has announced today completion of an ove...
Monika Safford (John J Kuiper Professor of Medicine and Chief of General Interna...
Every 21 seconds someone in the USA calls Poison Control because of a medication...
Researchers have evaluated the long-term efficacy and safety of long duration du...
GE Healthcare and HeartFlow have entered into a global collaboration agreeme...
A stent coated with sildenafil (Viagra, Pfizer, and others) may someday help...
Radical changes to our health care system that take into account the unique need...
Medtronic has announced that the FDA has approved the company’s self-expandi...
Jonathan Afilalo (Division of Cardiology, Jewish General Hospital, McGill Un...
Medtronic will release the Resolute Onyx coronary stent system in Japan. Acc...
The American Medical Association (AMA) has issued a set of new Category III ...
Edwards Lifesciences has FDA approval for its Inspiris Resilia surgical aort...
A private cardiology clinic in Berlin, Germany, headed by Niels Jacobsohn ha...
Researchers at Georgia Institute of Technology and the Piedmont Heart Institute ...
Contracting shingles, a reactivation of the chickenpox virus, increases a pe...
The UK’s Information Commissioner’s Office (ICO) has found that the Royal Fr...
LifeTech, at the 2017 Congenital and Structural Intervention Congress (CSI; 28 J...
Breast implants may disrupt an electrocardiogram (ECG) and could result in a...
A sudden catastrophic loss of heart function, or cardiac arrest, occurred signif...
A new study from the Women in Innovation Initiative and Drug-Eluting Stents (WIN...
Ravi S Hira (Section of Cardiology, University of Washington, Seattle, USA) ...
The British Heart Foundation has produced a video to help the general public und...
Getinge has announced the opening of a new modern hybrid operating room (OR) at ...
A study published in the Journal of Interventional Cardiology indi...
Mitralign has announced the start of its SCOUT II study in Europe; the study is ...
Philips and Spectranetics have announced that they have entered into a definitiv...
New data presented, by Luca Testa (Department of Cardiology, IRCCS Policlini...
With our population rapidly ageing and improvements in care in other conditi...
Young adults with a history of asthma are at a greater risk of thickening of the...
A worldwide recall of Venture catheters has been voluntarily initiated by its Te...
Novartis has announced topline results from the global Phase III CANTOS study in...
A review of the neurological complications that occurred after sur...
Women and their physicians are largely uneducated when it comes to females and h...
TriReme Medical, a subsidiary of QT Vascular, has announced that its angiogr...
A study, published in EuroIntervention, provides further evidence that there...
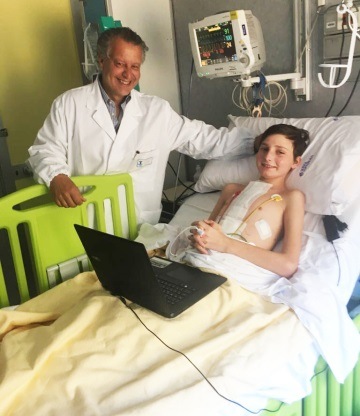
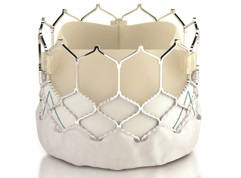
A 12-year-old boy at Bambino Gesù Children’s Hospital in Rome, Italy, has be...
Data from the TOPIC (Timing of platelet inhibition after acute coronary synd...
Public Health England (PHE) and the Royal Society for Public Health (RSPH) have ...
The United States Food & Drug Administration (FDA) has granted 510(k) cleara...
Most of the initial registries, and the large randomised trials, for transca...
Alexander C Fanaroff (Division of Cardiology, Duke University, Durham, USA) ...
The ARTE study, which was simultaneously presented at EuroPCR (16–19 May, Pa...
The District Court in Munich, Germany has partially found in favour of Edwar...
BioTrace Medical announced that its Tempo temporary pacing lead was featured...
Emmanuel Messas (Hôpital Européen Georges Pompidou, Paris, France) and colle...
Venus Medtech has purchased InterValve, which sells balloon aortic valvuloplasty...
Claret Medical has announced that several of the largest centres of excellen...
Sex-specific cardiovascular drug dosages are needed to reduce adverse reacti...
Peter Rothwell (University of Oxford, Oxford, UK) and colleagues report in T...
A pooled patient-level analysis of patients undergoing physiological assessm...
According to a study presented at EuroPCR (16–19 May, Paris, France), compar...
Sameer Arora (Division of Cardiology, University of North Carolina at Chapel...
A new study suggests that a novel skin dose-tracking system (Toshiba) may re...
The first patients in an early feasibility study of HLT’s transcatheter aortic v...
OrbusNeich has announced the launch of its Sapphire percutaneous coronary angiog...
On 16 September 1977, Bernhard Meier helped Andreas Grüntzig to perform the ...
This year marks the 10th anniversary of the Transcatheter Valve Therapies (T...
Edwards Lifesciences has received US Food and Drug Administration (FDA) appr...
Claret Medical has received regulatory clearance from the FDA for its Sentin...
The first ever Inspiris Resilia aortic valve (Edwards Lifesciences) implanta...
Philips has announced the relaunch of the Pioneer Plus catheter, described a...
Micell Technologies has announced positive twelve-month data from its DESSOL...
HeartFlow has announced the appointment of Michael Buck as executive vice pr...
Singulex has been granted CE mark for its ultra-sensitive troponin assay (cTnl),...
In this PCR video, filmed during EuroPCR (16–19 May, Paris, France), Atul Pathak...
David Wald (Barts Heart Centre, St Bartholomew’s Hospital, London, UK) and h...
The US Food and Drug Administration (FDA) has cleared Stroke2prevent’s A-view de...
Statins are associated with improved heart structure and function, according to ...
Quitting methamphetamine use can reverse the damage the drug causes to the h...
Diesel pollution is linked with heart damage, according to research presented at...
BioVentrix has announced the first clinical use of its closed-chest Revivent...
According to a study published in the International Heart Journal, delivery ...
A study—AIDA (Amsterdam investigator-initiated Absorb strategy all-comers tr...
Vinayak—“Vinnie”—Bapat (New York Presbyterian Hospital/Columbia Medical Center...
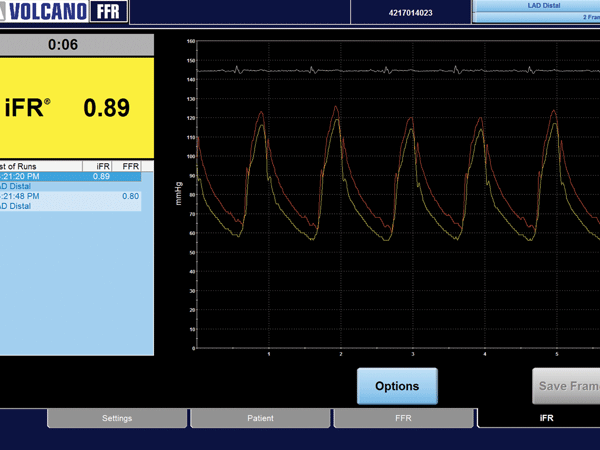
The top story this issue (45; May 2017) reviews whether iFR, following the resul...
The top story this issue (45; May 2017) reviews whether iFR, following the resul...
ACIST Medical Systems announced results from the ACIST-FFR study (Assessment...
Data for the Resolute Onyx drug-eluting 2mm stent (Medtronic), which were p...
According to late-breaking trial presented at EuroPCR (16–19 May, Paris, Fra...
Data presented at EuroPCR (16–19 May, Paris, France) indicate that a metalli...
Gore has announced positive results from the REDUCE study, which is assessing th...
Data from the REPRISE III trial indicate that the Lotus transcatheter aortic val...
Teleflex showcased its complex percutaneous coronary intervention (PCI) product ...
In patients undergoing transradial primary percutaneous coronary intervention (P...
Data presented from ABSORB China and ABSORB Japan at EuroPCR (16–19 May, Par...
New analyses from the PLATINUM Diversity study underscore the need for great...
Boston Scientific Corporation has announced the close of its acquisition of ...
New positive data on the self-expanding Medtronic CoreValve platform from th...
Drug-eluting stent technology with durable polymer has shown encoura...
Abiomed has announced the debut of the third generation Impella CP heart pum...
Boston Scientific has announced the schedule of key data presentations, incl...
NaviGate Cardiac Structures has announced that the Gate catheter-guided tricuspi...
A large nuclear cardiology laboratory has slashed its average radiation dose by ...
Despite a constant evolution in stent/scaffold design, stent failure is stil...
Preadmission clinics for patients awaiting elective procedures or surgery ar...
A new study indicates that the apparent survival benefit after transcatheter...
GE Healthcare announced that it has received an indication approval from the...
LivaNova has announced it has acquired the remaining outstanding interests in Ca...
Ambarish Pandey (Division of Cardiology, University of Texas Southwestern Me...
The ENVISAGE-TAVI AF is the first study to evaluate the effects of a novel o...
Teleflex has announced 510(k) clearance from the FDA for its AC3 Optimus int...
Abiomed announced today the enrolment of the first patient in the FDA approved p...
Philips has announced the results from a global study indicating that automated ...
LivaNova has announced the presentation of data from multiple studies demons...
The FDA has approved Medtronic’s Resolute Onyx drug-eluting stent, which the...
Having a non-O blood group is associated with a higher risk of heart attack, acc...
The US Food and Drug Administration (FDA) has classified Medtronic’s recentl...
A meta-analysis of single-centre studies indicates that the use of the trans...
A new study indicates that patients with glycosylated haemoglobin A (HbA1c) ≥7 a...
Abbott Vascular has written a letter to European physicians to say that from...
Gillian Jessurun (Treant Zorggroep, Scheper Hospital Emmen, The Netherlands) has...
Cordis has received the CE mark for its new Railway sheathless access system...
Preliminary results from the Medtronic -sponsored HVAD LATERAL study have de...
Results of the TARGET All Comer (AC) clinical trial will be released at annual...
Occlutech has obtained CE mark approval for its Perimembranous ventricular septa...
Medtronic’s ENDURANCE Supplemental trial of the company’s HVAD ventricular a...
HLT has announced that the first patient implants in a feasibility clinical stud...
NVT has received the CE mark for its Allegra transcatheter aortic valve, whi...
Reva has been granted CE mark approval for its Fantom drug-eluting bioresorb...
Insomnia is associated with increased risk of heart attack and stroke, according...
Espero Pharmaceuticals and Armetheon are to merge to form a new company. Upon co...
Boston Scientific Corporation have definitively agreed to acquire Symetis, a pri...
This year’s SynCardia awards recognise leadership in the Destinati...
Biotronik has released details of the clinical performance of its Magmaris m...
Cardiovascular Systems and the Cardiovascular Research Foundation (CRF) have...
iVascular has obtained CE mark for the thrombus extraction catheter Capturer...
The SURTAVI trial indicates that transcatheter aortic valve implantation (TA...
REVA Medical has announced that it has been granted approval to conduct an e...
The GEMINI-ACS study, which was simultaneously presented at the 2017 scienti...
Daniel P Andersson (Department of Medicine, Karolinska Institutet, Unit of E...
At JIM 2017 (9-11 February, Milan, Italy), we spoke to Bernardo Cortese (Interve...
Evan Shlofmitz (Northwell Health, Manhasset, USA) presented the results of t...
Tryton Medical has announced that the first US commercial case using the Try...
A new meta-analysis presented this week at the American College of Cardiolog...
The FDA has approved Medtronic’s CoreValve Evolut Pro transcatheter aortic v...
Cardiologists can now access the advanced ultrasound imaging technology need...
Siemens Healthineers and HeartFlow are to collaborate on joint-solution comp...
Amgen has announced detailed results from the evolocumab (Repatha) cognitive...
Unfors RaySafe has introduced the RaySafe i3, to its suite of real-time dosime...
The Medicines Company and Alnylam Pharmaceuticals have announced positive final ...
Of 84 older patients undergoing transcatheter aortic valve implantationt (TA...
The 12-month results of Biotronik’s BIOFLOW-IV multicentre clinical study ha...
Two US Food and Drug Administration-cleared medical devices designed to remove p...
New recommendations have been published by the American Heart Association and th...
Writing in The New England Journal of Medicine, Deepak L Bhatt (Brigham and ...
The Tsimane people—a forager-horticulturalist population of the Bolivian Ama...
Acarix has announced the results from a new multicentre trial of its handh...
Mary Norine Walsh has become president of the American College of Cardiology...
Researchers at Cleveland Clinic, Cleveland, USA, put five popular wrist-worn fit...
Community coffee shops and cash machines might be ideal locations for public acc...
As the Zika virus continues to spread globally, new evidence has emerged abo...
Late-breaking clinical research presented at the American College of Cardiol...
Patients with heart failure in the USA are more likely to be hospitalised and mo...
Data for a new drug that inhibits proprotein convertase subtilisin-kexin typ...
Two-year data from the ABSORB III study indicate that a bioresorbable vascul...
Sung-Han Yoon (Cedars-Sinai Heart Institute, Los Angeles, USA) and others re...
Results from the SURTAVI clinical trial, which were presented today at the 2...
Vascular Graft Solutions has announced interim results of VEST III, a post-m...
Centurion Medical Products has launched its “smART” kit for radial arterial...
Innovative Cardiovascular Solutions has announced the successful f...
A study published in European Heart Journal: Acute Cardiovascular Care indic...
A study, published in the Journal of Interventional Cardiology, indicates th...
Francesco Costa (Swiss Cardiovascular Center Bern, Bern University Hospital,...
SynCardia, manufacturer of the world’s first and only FDA, Health Canada and...
Following FDA approval, Medtronic’s Melody transcatheter pulmonary heart val...
According to a study published in European Heart Journal–Cardiovascular Phar...
A new study published in Circulation Research finds that the use of haemodyn...
The American College of Cardiology (ACC), along with several partnering orga...
Everolimus-Eluting Platinum Chromium Coronary Stent System
Drug Eluting s...
MR Solutions’ new 9.4 tesla cryogen-free preclinical scanners was demonstrated t...
Rules governing the conduct of clinical trials are failing to produce the intend...
The Patents Court in the UK has determined that one of Boston Scientific’s p...
Alvimedica’s Illumina study has completed enrolment. The Illumina study has been...
The US Food and Drug Administration (FDA) has approved Tryton Medical’s Prem...
According to a review published in JACC: Cardiovascular Imaging, the simple comp...
The first patient has been enrolled into Daiichi Sankyo’s ENTRUST-AF PCI study. ...
Teleflex incorporated has announced 510(k) clearance by the US Food and Drug Adm...
The second clinical case using Micro Interventional Devices’ proprietary MIA (mi...
LivaNova has applied for the voluntary cancellation of its standard listing of o...
Johns Hopkins Medicine, Baltimore, USA, the Maryland Stem Cell Research Fund (MS...
Only 16% of heart attack survivors get the recommended amount of physical activi...
Elsevier and the American Society of Echocardiography has announced the launch o...
The benefits of physical activity may outweigh the impact of overweight and obes...
Corindus Vascular Robotics has entered into a Securities Purchase Agreement ...
Cardiovascular Systems (CSI) has announced the addition of independent board mem...
Physicians should be well-versed in the herbal medications heart disease patient...
Following current dietary recommendations may lead to small improvements in ...
While lifestyle patterns, including physical activity and body mass index (BMI),...
How are smartphones and computer programs transforming healthcare, especiall...
A new meta-analysis, published in JACC: Cardiovascular Interventions, indica...
The FDA has approved a nano-coated coronary stent system—CeloNova Bioscience...
A new study published in Catheterization and Cardiovascular Interventions in...
This educational supplement is only for readers in countries outside E...
A network meta-analysis, published in the Journal of the American College of...
FDA 510(k) clearance has been granted to BTG’s EKOS control unit 4.0. A press re...
In an open public hearing of the FDA’s circulatory system devices panel, acc...
Philips has announced the global launch of Azurion—its next-generation image...
A new study published in JACC: Cardiovascular Interventions indicates that the t...
According to Reuters, Boston Scientific is recalling all ranges of its trans...
Teleflex Incorporated has completed its previously announced acquisition of Vasc...
Cardiovascular Systems (CSI) has released one-year results from its Coronary Orb...
Ki Hong Choi (Division of Cardiology, Department of Medicine, Cardiac and Va...
Biosensors has announced that the first patient has been enrolled in LEADERS...
Biotronik’s Pro-Kinetic Energy bare metal stent has received FDA approval, w...
A study, published in Catheterization and Cardiovascular Interventions, foun...
Researchers have projected that aggressively lowering blood pressure could help ...
The German Institute for the Hospital Remuneration System (InEK) has renewed...
Symetis, a medical technology company specialising in the development, manufactu...
Medtronic has announced the launch of Medtronic Impact in the Europe, Middle Eas...
In Issue 44 (February 2017), we review why EXCEL and NOBLE had such different fi...
In Issue 44 (February 2017), we review why EXCEL and NOBLE had such different fi...
The UK’s National Institute for Health and Care Excellence (NICE) has issued gui...
A new test that predicts an individual's risk for developing cardiovascular dise...
Rebecca Hahn (Center for Interventional Vascular Therapy, Columbia Universit...
In the largest epidemiological study conducted in the developing world, research...
Medtronic has launched the DxTerity diagnostic angiography cathete...
The first commercial procedures using Corindus Vascular Robotics’ CorPath GRX sy...
Armetheon has reached agreement with the US Food and Drug Administration (FDA) f...
The phase three COMPASS trial, evaluating the efficacy and safety of rivaroxaban...
An anticancer agent in development has been shown to promote regeneration of...
The US Food and Drug Administration (FDA) has granted Angionetics “Fast Track” d...
Cardiovascular systems (CSI) presented six-month data from its LIBERTY 360 d...
In response to a growing body of evidence of the existence of "covert" brain...
Veryan Medical has received a further £13.5m of funding in the form of both ...
The idea of a fully bioresorbable scaffold is attractive as the need for mec...
Gennaro Giustino and others report in the Journal of American College of Car...
Functional tricuspid regurgitation is increasingly recognised as an importan...
The FOURIER trial evaluating whether evolocumab (Repatha, Amgen) reduces the...
According to a SeekingAlpha transcript of an Edwards Lifesciences fourth-qua...
Multiple sites in Spain have begun enrolling patients in the DuraGraft coronary ...
Ryan D Madder (Frederik Meijer Heart & Vascular Institute, Spectrum Health, ...
In a research letter published in the Journal of the American College of Car...
Because of a significant improvement in the prehospital treatment of patient...
Northwestern Memorial Hospital has been ranked best in the USA for survival of p...
The number of adults living with heart failure increased from about 5.7 mill...
The Society for Cardiovascular Angiography and Interventions (SCAI) has name...
Edwards Lifesciences has closed its previously announced acquisition of Valt...
Elderly patients with aortic stenosis and medium surgical risk experienced bette...
In this video, by Heart Valve Surgery, Paul Powers describes his life before and...
Terumo has announced that it has completed its acquisition of certain assets...
Roche has received US Food and Drug Administration (FDA) 510(k) clearance for it...
Sonny Palmer (St Vincent’s Hospital, Melbourne, Australia) and others report...
The RADIATION (Radiation dose in percutaneous coronary procedures through tr...
Alvimedica has received the CE mark for its new polymer-free amphilimus-eluting ...
The CE mark has been granted to Medtronic for the 34mm-valve version of its ...
Direct Flow Medical, which markets a CE-marked transcatheter aortic valve implan...
Robert Califf is to step down as US Food and Drug Administration (FDA) commi...
Shingo Kuwata (University Heart Center, Zurich, Switzerland) and others repo...
A medical team from Clínica Universidad de Navarra in Pamplona, Spain, has s...
Heightened activity in the amygdala has been associated with a greater risk of h...
Following the recent announcement from the American College of Cardiology (ACC) ...
According a news report on Mass Device, Boston Scientific has “found a fix” ...
ACIST Medical Systems has announced that enrolment has been completed in its ACI...
The American College of Cardiology (ACC) has named David J Moliterno (Jack M Gil...
Ryo Yanagisawa (Department of Cardiology, Keio University School of Medicine...
OrbusNeich has launched the Scoreflex NC, a non-compliant scoring balloon target...
A new expert consensus decision pathway will assist clinicians and hospitals in ...
Arterys has received 510(k) clearance from the US Food and Drug Administrati...
St Jude Medical has announced that it will immediately deploy the latest release...
The Medicines Company has announced positive top-line results from the interim a...
The Women in Cardiology (WIC) section of the American College of Cardiology ...
BioCardia has announced the initiation of its CardiAMP heart failure pivotal tri...
MicroPort Medical has recently completed the patient enrolment of the pre-market...
Somahlution has announced enrolment of the first patient in its DuraGraft corona...
Abbott Vascular has completed its acquisition of St Jude Medical. This follows r...
A new study indicates that “transcatheter valve-in ring”—implanting a transc...
Stephen Ramee (Ochsner Medical Center, New Orleans, USA) and others outline ...
NaviGate Cardiac Structures has announced that a novel valved stent that can cap...
Twelve-month clinical results from Medinol’s BIONICS study have demonstrated tha...
The American College of Cardiology (ACC), along with several partnering organisa...
Women with significant aortic valve disease who undergo transcatheter aortic...
A new report from the American College of Cardiology and the American Heart Asso...
Micro Interventional Devices has successfully completed the first clinical impla...
A high-sensitivity blood test could be used to predict which patients are at ris...
A new study, published in The BMJ, indicates that for centuries, children ha...
The US Food and Drug Administration (FDA) has issued a final rule to ban pow...
The UK’s National Institute for Health and Care Excellence (NICE) has announ...
The association between infections and acute coronary syndromes has been the...
The new European guidelines on cardiovascular disease prevention in clinical...
Recently, there has been a move towards transcatheter aortic valve implantat...
Terumo has reached an agreement with Abbott and St Jude Medical to acquire certa...
Patients with acute coronary syndrome may be at an increased risk for suicide co...
Boston Scientific Corporation has closed its acquisition of certain manufact...
AngioSoma has appointed Ken Stephenson as chief executive officer, board member,...
Abiomed has expanded its FDA pre-market approval for Impella heart pump use ...
4Tech has appointed Michael Ennen as president and chief executive officer, effe...
Teleflex is to acquire Vascular Solutions in a transaction valued at approximate...
Simon Wilson (Royal Infirmary of Edinburgh, Edinburgh, UK) and oth...
Musa A Sharkawi (Department of Cardiovascular Medicine, Hartford Hospital, H...
Medtronic has been granted reimbursement approval from the Japanese Ministry...
Boston Scientific has agreed to acquire certain manufacturing assets and cap...
Cardiac Dimensions has received FDA approval for its investigational device exem...
Ran Kornowski (Department of Cardiology, Cardiac Catheterization Laboratorie...
Medtronic’s HVAD system left ventricular assist device (LVAD) has received t...
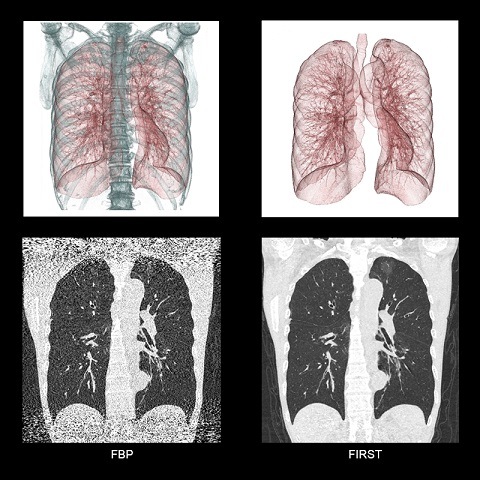
Toshiba Medical’s Forward projected model-based iterative reconstruction sol...
Amsel Medical Corporation is to present initial animal results of its “A Novel M...
In Catheterization and Cardiovascular Interventions, Lloyd Klein (Rush Medic...
Valtech Cardio has agreed to be acquired by Edwards Lifesciences. The acquis...
Neovasc has received both regulatory and ethics committee approval to initia...
At the 2016 meeting of the Radiological Society of North America (RSNA; 27 Novem...
With the introduction of the AquilionTM ONE/GENESIS Edition, the new system from...
OrbusNeich launched its latest generation dual therapy stent, the Combo Plus...
Admedus has announced that, in collaboration with its regional partner Genph...
BioVentrix has announced the first clinical use in the Netherlands of its cl...
Valentin Fuster (director of Mount Sinai Heart and physician-in-chief of The Mou...
It is virtually impossible to remove all contamination from robotic surgical ins...
The new St. Jude Medical™ Trifecta™ valve with Glide™ Technology (GT) featur...
SyncVision (Philips Volcano) enables co-registration between angiogram and i...
The results of the FUTURE (Functional testing underlying revascularisation) ...
A new study, by researchers from the Intermountain Medical Center Heart Inst...
HeartFlow has announced the approval of its HeartFlow FFRCT analysis by the ...
Two drug regimens involving low doses of rivaroxaban (Xarelto, Bayer)—one wi...
The American Heart Association (AHA) awarded its Basic Research Prize for 20...
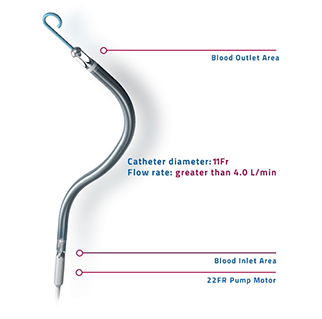
The results of the MOMENTUM 3 US IDE clinical study, which were presented at...
Corvia Medical has announced one-year follow-up data from the REDUCE LAP-HF clin...
Ehrin J Armstrong (Section of Cardiology, Denver VA Medical Center and Unive...
The Medicines Company and Alnylam Pharmaceuticals have announced positive result...
Mitralign has announced the successful compassionate use treatment of a patient ...
Amgen has announced that adding its PCSK9 inhibitor evolocumab (Repatha) to ...
The Cardiovascular Research Foundation (CRF) is to launch a new internationa...
Patient enrolment into the international Phase IIIb RE-DUAL PCI st...
Terumo Europe has entered into a distribution agreement with New Zealand com...
Highlights: Lower revascularisation rate means drug-eluting stents are still pre...
Highlights: Lower revascularisation rate means drug-eluting stents are still pre...
According to the three-year results of the ABSORB II trial, a bioresorbable ...
Two randomised controlled trials have produced what appears to be conflictin...
Final results from the RESPECT trial found that percutaneously closing a pat...
A study in JACC: Cardiovascular Interventions indicates that patients who requir...
Pfizer is to discontinue the global clinical development program for bococizumab...
Merit Medical has received 510(k) clearance for its SwiftNinja steerable microca...
Xenios AG has become part of Fresenius Medical Care. A press release reports tha...
Svelte Medical Systems has announced the start of enrolment in the DIRECT II...
The National Institute of Health and Care Excellence (NICE) has published a fina...
Mitralign presented 30-day data from the SCOUT I study, which designed to ev...
Data presented at the 2016 Transcatheter Cardiovascular Therapeutics (TCT) m...
Neovasc has announced today its notable presentations at the 2016 Transcathe...
Medtronic has unveiled new clinical data that shows that patients treated wi...
UK charity Heart Valve Voice has launched a report—Towards a Heart Healthy Futur...
Six-month clinical data for Elixir Medical’s bioresorbable scaffold (Desolve) we...
At the 2016 Transcatheter Cardiovascular Therapeutics (TCT) meeting (29 Octo...
Arterys has received 510(k) clearance from the FDA for its Arterys Software. Thi...
Arrhythmia Alliance, the UK-based heart rhythm charity, is calling on NHS En...
A study presented at the last late-breaking trial session of the 2016 Transc...
Data from the PLATINUM DIVERSITY trial, which was presented at the 2016 Tran...
The SENTINEL trial, which was presented at the 2016 Transcatheter Cardiovasc...
At a symposium at the 2016 Transcatheter Cardiovascular Therapeutics (TCT) m...
Results of the REVELUTION study, simultaneously presented at the 2016 Transc...
Today, Medtronic presented new positive data from two large registries aimed...
PCR London Valves 2016 opened in the main arena with the Edwards Lifescience...
The FDA has approved St Jude Medical’s Amplatzer patent foramen ovale (PFO) ...
BIO-RESORT, presented at TCT today during a late-breaking trial session, has fou...
ILUMEN III, the first late-breaking to be presented at the 2016 Transcathete...
Tryton Medical and Cardinal Health have announced that they have established...
Gabor Toth (University Heart Centre Graz, Graz, Austria), Bernard De Bruyne ...
Valtech Cardio will present a symposium on mitral and tricuspid valve repair...
According to a study by researchers from the University of Turku (...
Corindus Vascular Robotics has received 510(k) clearance from the FDA for it...
Mitralign is to outline data for its Trialign tricuspid repair system at the 201...
BioTrace Medical has received FDA 510(k) clearance for its Tempo Lead, which...
St Jude Medical has announced that, based on the preliminary voting results from...
Medtronic has announced the FDA approval and US launch of the CoreValve Evol...
Miracor Medical Systems has raised additional funding as part of its Series-...
The latest image guidance solutions of Philips will be featured at Transcath...
A new study, published in JAMA Cardiology, indicates that health s...
Anna Franzone (Department of Cardiology, Swiss Cardiovascular Center, Univer...
CeloNova BioSciences has provided details of its activities at the 2016 Transcat...
Boston Scientific has announced key data that will be presented at the 2016 ...
Svelte Medical has received the CE mark for the Direct sirolimus-eluting coronar...
New data for St Jude Medical’s devices and technologies will be presented du...
A study published in Circulation has not only found that patients who adhere...
Understanding how aggressively to treat patients in their last years of life...
The FDA has granted conditional approval to conduct an investigational devic...
Medtronic Canada has announced it has received a Health Canada licence for i...
Vascular Solutions has received 510(k) clearance from the FDA for the Fluent inf...
Data from the INTERHEART study, published in Circulation, supports the theor...
Elixir Medical has announced that it will showcase the first live case demonstra...
Corindus Vascular Robotics is to host a breakfast symposium entitled “Roboti...
Cardiologists at The James Cook University Hospital (Middlesbrough, UK), after r...
The Leonhard Lang defibrillation electrode DF29N is being recalled due to a ...
Robert Wang (The Heart and Vascular Institute, Cleveland Clinic, Cleveland, ...
Data from the interim analysis of the ongoing ORION-1 study, which is evaluating...
Abbott and St. Jude Medical have announced an agreement in principle to sell...
Tryton Medical has announced that it will be sponsoring a clinical symposium...
St Jude Medical is to form a Cyber Security Medical Advisory Board (CSMAB) to ad...
According to a study published in JACC: Cardiovascular Interventions, patients u...
Corindus Vascular Robotics has announced that Suzette Jaskie has been appointed ...
Lauren Sinnenberg (@lsinnie Penn Medicine Social Media and Health Innovation...
A new study indicates that patients with mild-to-moderate chronic kidney dis...
Samir B Pancholy (The Wright Center for Graduate Medical Education, The Comm...
BioStable Science & Engineering has completed a direct de novo applicati...
At the South Africa Heart Association annual congress (8–11 September, Cape ...
St Jude Medical has announced the FDA clearance and launch of its PressureWi...
A novel device for treating tricuspid regurgitation has become the 2016 reci...
Ian Meredith, currently professor of Medicine and Cardiology for Monash Univ...
Kieran Sandwell, who was born with transposition of the great arteries, underwen...
CVRx has appointed Tom Moore to the newly created, officer position of vice ...
Shivan J Mehta (Department of Medicine, Perelman School of Medicine, Univer...
Interim analysis results from ORION-1, which is evaluating The Medicines Company...
Medtronic has reported that two previously communicated global voluntary rec...
Somahlution announced the presentation of new data showing the its DuraGraft vas...
The MAJESTIC trial set out to evaluate the performance of the Eluvia paclitaxel-...
A study published in the American Journal of Cardiology indicates that 94% o...
Positive clinical data for Medtronic’s investigational Avalus pericardial aortic...
Xeltis has announced that three pediatric patients have been successfully im...
Edwards Lifesciences has received the CE mark for its Inspiris Resilia aorti...
The NORSTENT (Norwegian coronary stent trial) study, which was presented at ...
Francesco Maisano (University Hospital Zurich, Zurich, Switzerland) led a te...
Abiomed has announced that its heart pumps Impella 2.5 and Impella 5.0 have ...
Royal Philips has announced a new partnership with the World Heart Federation (W...
Opsens has announced 510(k) clearance from the US Food and Drug Administrati...
It has become commonly accepted that women do worse than men following a heart a...
Increasing stomach fat—especially abdominal visceral fat—has been associated...
Xeltis AG, a clinical-stage medical device company developing the first-ever...
A first-in-man study indicates that percutaneous edge-to-edge repair with Ab...
Although much of the innovation in transcatheter solutions for structural he...
A study published, as a research letter, in the Journal of the American College ...
Preliminary real-world findings, presented at PCR London Valves (18–20 Septe...
The Phase 3 GLAGOV (Global assessment of plaque regression with a PCSK9 anti...
Claret Medical has announced its filing of a marketing application with the ...
Following last month’s FDA approval for the expanded indication of Sapien 3 ...
4Tech’s transcatheter tricuspid valve repair device (TriCinch) has been used for...
Coinciding with PCR London Valves (18-20 September, London, UK), Boston Scie...
Valtech Cardio will present the first-in-human data for its Cardioband Tricu...
Caisson Interventional has announced the first successful human implants of its ...
Recent studies have indicated that complete revascularisation in patients wi...
Data from a new study, BASKET-SAVAGE, indicate that patients who receive a d...
FineDuo—a low profile, multifunctional, dual-lumen microcatheter—now has the...
BTG has launched the βETA Radiation Safety Programme, a new initiative designed ...
Data from the SENTINEL pivotal IDE trial will be presented during a late-bre...
The Cardiovascular Research Foundation (CRF) has announced the 11 late-break...
Marco Barbanti (Ferrarotto Hospital, University of Catania, Catania, Italy) ...
At PCR London Valves (18–20 September, London, UK), Holger Eggebrecht (Cardi...
A study presented at the 2016 European Society of Cardiology (ESC) congress ...
A team of clinical scientists at the University of Bristol (Bristol, UK) hav...
European standards to prevent repeat heart attacks have been published in th...
A placebo-controlled, double-blind trial—NACIAM (N-acetylcysteine in acute m...
Implantable cardioverter-defibrillators (ICDs) do not improve overall long-term ...
Berlin Heals has closed a second investment round in the run-up to the first-in-...
Results from the first study to compare outcomes of two left atrial appendag...
The initial results of the first randomised controlled trial—PRAGUE 18—to di...
Pope Francis visited the 2016 European Society of Cardiology (ESC) congress (27-...
Wanting to be like his father, Philip Urban (Cardiovascular Department, Hôpi...
Highlights: Study indicates signal for valve degeneration in TAVI patients by ei...
Highlights: Study indicates signal for valve degeneration in TAVI patients by ei...
Vivasure Medical has announced that the company has completed a series C financi...
In new research published in PLOS Biology, University of California, Irvine (Irv...
Medtronic has been recognised as one of the world's leading companies for su...
The St Jude Medical Amplatzer Amulet Investigational Device Exemption (IDE) ...
Opsens, which has an advanced optical-based pressure guidewire (OptoWire) that a...
Results from the ANTARCTIC study, which was simultaneously presented at the ...
A new analysis of data from the CHAMPION PHOENIX (Cangrelor versus standard ...
Women have a 50% higher chance than men of receiving the wrong initial diagnosis...
A University of British Columbia (Vancouver, Canada) invention has made it p...
The results of NORSTENT (Norwegian coronary stent trial) indicate that secon...
Low socioeconomic status has been associated with a higher risk of a second ...
The European Society of Cardiology has launched a novel position paper, under th...
Royal Philips has launched the IntelliSpace Cardiovascular 2.1, the latest versi...
The Mediterranean diet has been associated with a reduced risk of death in p...
The first European Society of Cardiology (ESC) Guidelines on Atrial Fibrillation...
Rose Medical Center has become one of the first hospitals in Colorado (USA) ...
Medicure has received approval from the US Food and Drug Administration (FDA) fo...
According to a study published in JAMA Cardiology, among those 18–55 years, ...
Nicolas Meneveau (Besancon, France) told delegates at the 2016 European Soci...
The number of cardiovascular drugs in the research pipeline has declined across ...
The NIPPON (Nobori dual antiplatelet therapy as appropriate duration) study, pre...
Zoll Medical has announced that results from the largest German registry of ...
There is just a one in five chance that a potentially life-saving ...
Physiological assessment with fractional flow reserve (FFR) is an established ap...
A press release reports that Royal Philips will be showcasing its latest in ...
Medtronic has completed its acquisition of HeartWare International—a develop...
According to a study published in the Annals of Thoracic Surgery, the Zip su...
This educational supplement is only for readers in countries outside France, Jap...
A pre-specified analysis of PRODIGY (Prolonging dual antiplatelet treatment ...
Apurva Motivala (Division of Cardiology, Columbia University, New York, USA)...
In this supplement,* Cardiovascular News reviews how unique imaging technologies...
In this CTSNet video, filmed at the 2016 STS Annual Meeting (Phoenix, USA), Piet...
Long-term data from a Phase II extension study for the repair of congenital ...
An American College of Cardiology (ACC) press statement reports that the Cen...
Edwards Lifesciences has received FDA approval for its advanced Intuity Elite ra...
OrbusNeich has announced that two major clinical studies have now completed ...
Edwards Lifesciences has announced US Food and Drug Administration (FDA) app...
Analysts from Technavio have forecast the global renal denervation market to gro...
Corindus Vascular Robotics and Acist Medical Systems have come together to p...
Spectranetics has been granted the CE mark for its AngioSculptX Drug-coated perc...
Tim Kinnaird (Department of Cardiology, University Hospital of Wales, Cardif...
A group of cardiovascular researchers have formed a consortium—Academic Rese...
InSeal Medical has received CE mark approval for its InClosure VCD; a large ...
Advances in technology coupled with an increased use of social media and per...
Royal Philips, along with several other organisations, is to collaborate with th...
Boston Scientific has sent a letter to its customers notifying them that it ...
Corindus Vascular Robotics has announced that J Aaron Grantham, an intervent...
Essential Medical has received investigational device exemption (IDE) approval f...
Transcatheter mitral valve implantation (TMVI) is being explored as an approach ...
Medtronic has received CE marking for the self-expanding, recapturable and rep...
Hanna E Bloomfield (Minneapolis VA Medical Center, Minneapolis, USA) and oth...
Since its introduction into clinical practice, transcatheter aortic valve im...
Micro Interventional Devices has received FDA market clearance for its Per...
In revised recommendations, the American Academy of Neurology (AAN) states that catheter-based closure should not be routinely recommended for people who have had a stroke and also have patent foramen ovale (PFO). The practice advisory, which updates a previous AAN guideline, has been published in Neurology.
Miguel Haime (VA Boston Healthcare System and Boston Medical Center, Boston, USA) is to present an abstract about Somhulation's DuraGraft during a rapid response session at the 2016 annual meeting of the European Association for Cardio-Thoracic Surgery (EACTS; 1–5 October, Barcelona, Spain).
After receiving clearance from the US Food & Drug Administration (FDA) earlier this year for expanded Indications for Use with the company's patented technology, ClearFlow has developed a new model of the PleuraFlow product for the paediatric market.
According to an email from the consumer watchdog, the document is intended to clarify "how the FDA determines that real-world data may be sufficient for use in premarket and postmarket regulatory decisions, without changing the evidentiary standards we use to make those decisions."
Providence Health Care has become the first centre in Canada to adopt the HeartFlow FFRct Analysis, and also first in the world to use the next generation version of the platform. The HeartFlow FFRct Analysis, which was recently approved by Health Canada, is a non-invasive technology used by clinicians to assess their patients for coronary artery disease.
Xenios AG, a developer of lung and heart assist therapies, has combined its novalung, i-cor, and medos websites into www.xenios-ag.com to further advance the Xenios platform. With the new Xenios website, the Heilbronn-based medical device company further advances its lung and heart assist therapy platform.
Stentys has started to enrol patients in its TRUNC trial, which is designed to evaluate the long-term safety and efficacy of the Xposition S stent in the treatment of unprotected left main coronary artery disease.
The data that Dvir presented at EuroPCR, as reported by Cardiovascular News, indicate that there is a significant increase in valve degeneration between five and seven years after a transcatheter aortic valve implantation (TAVI) device is implanted.
Speaking at EuroPCR (17–20 May, Paris, France), Danny Dvir reported that there is a significant increase in valve degeneration between five and seven years after a transcatheter aortic valve implantation (TAVI) device is implanted.
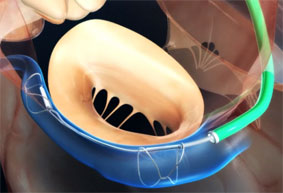
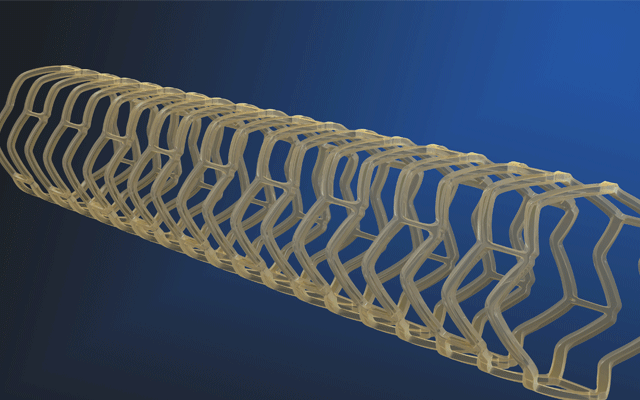
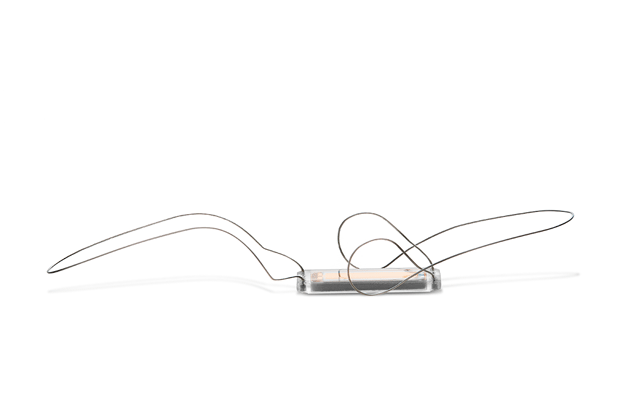
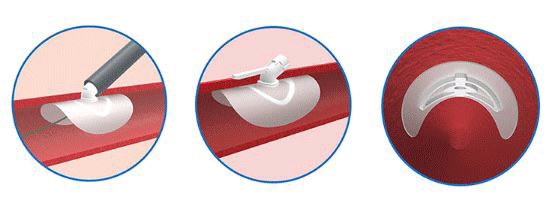
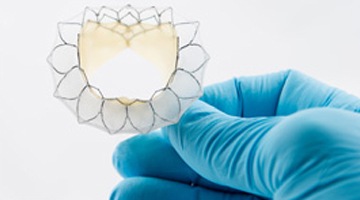
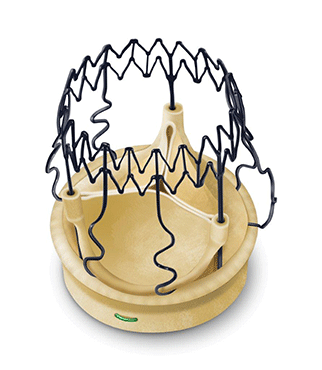
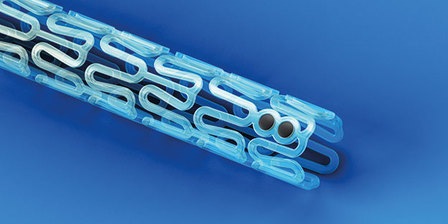
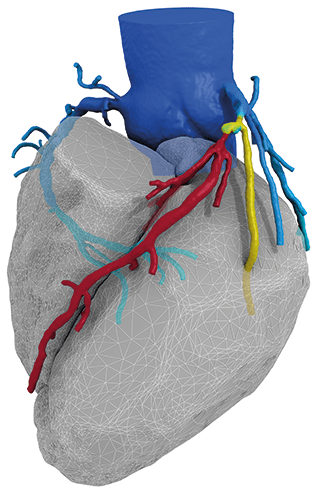
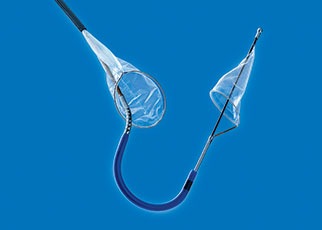
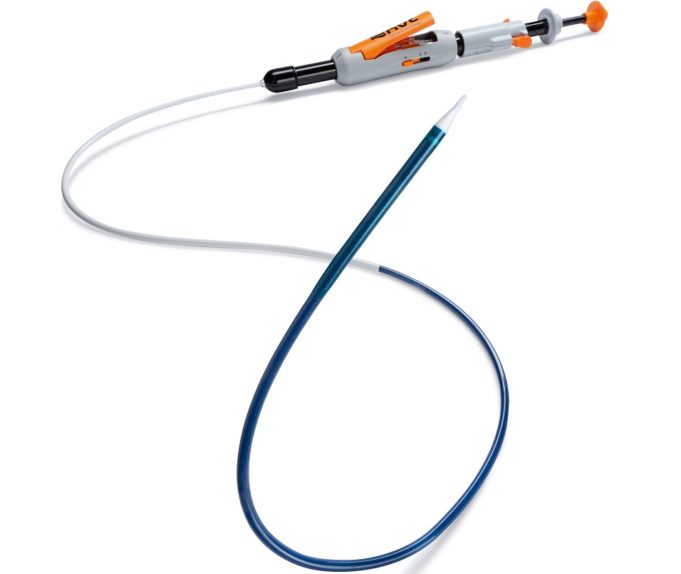
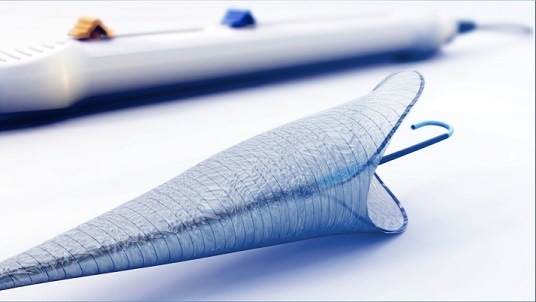
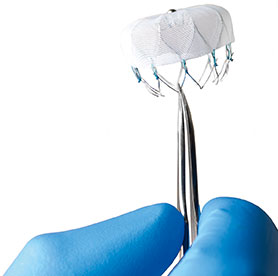
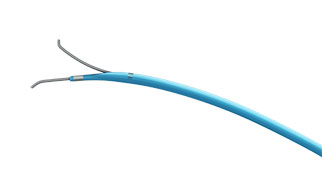
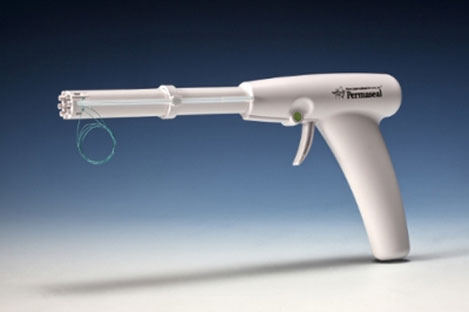
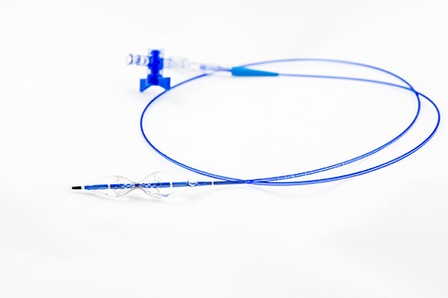
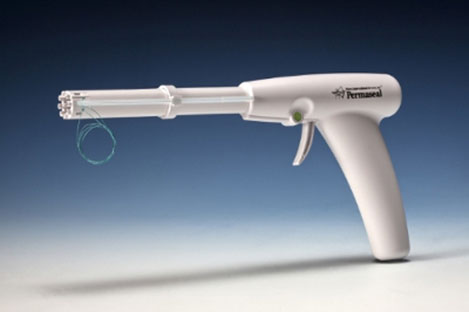
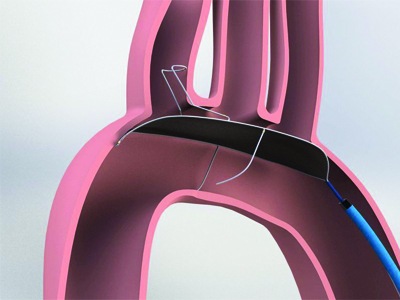
Essential Medical has received CE mark approval for Manta; its large bore vascular closure device. The device is a novel vascular closure device designed to close punctures ranging from 10F to 24F at femoral arterial access sites after cardiac catheterisation procedures such as TAVI.
A large cohort study, published in Catheterization and Cardiovascular Interventions, indicates that TAVI is associated with an overall 2% risk of upper gastrointestinal bleeding.
Results from the CORA-PCI (Complex robotically assisted percutaneous coronary intervention) indicate that robotic-assisted percutaneous coronary intervention (PCI), using the CorPath system (Corindus Vascular Robotics), is a safe and feasible approach to managing patients with complex lesions.
According to a study published in Open Heart, treatment with the Carillon device (Cardiac Dimensions) significantly reduces annular dimensions and improved mitral regurgitation, heart failure symptoms, and functional capacity in patients with functional mitral regurgitation.
Health Canada has approved Abbott's Absorb bioresorbable heart stent, making the device commercially available to treat people in Canada with coronary artery disease.
Commonly used medications and nutritional supplements may cause or worsen heart failure, according to the first scientific statement from the American Heart Association (AHA) to provide guidance on avoiding drug-drug or drug-condition interactions for people with heart failure.
Mitralign has announced that enrolment in the first phrase of its SCOUT study, which is evaluating percutaneous tricuspid repair with the Trialign system in patients with functional tricuspid regurgitation, has been completed.
Overall one-year survival was over 85% for high-risk or inoperable patients who underwent transcatheter aortic valve implantation (TAVI) with Sapien 3, according to a study published in Circulation.
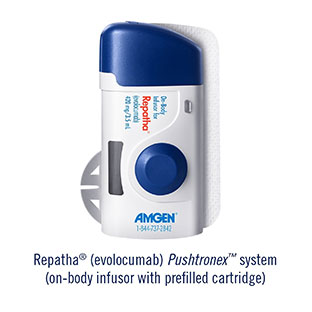
Amgen has received FDA approval for its Repatha (evolocumab) Pushtronex system (on-body infusor with prefilled cartridge), a new, monthly single-dose administration option. The Pushtronex system is a hands-free device designed to provide 420mg of Repatha in a single dose.
Sunshine Hearth has updated its clinical strategy, which it says could benefit an under-served population of patients with Class III heart failure and other related conditions. The company is moving forward with a therapeutic strategy focused on neuromodulation rather than counterpulsation.
Essential Medical has received IDE approval from the FDA to begin the US clinical trial of its large bore vascular closure device (Manta). The study will evaluate the safety and efficacy of vascular access closure using Manta for femoral arterial access site.
A study published in the Journal of the American College of Cardiology (JACC) indicates that patients who develop heart failure after their first myocardial infarction have a greater risk of developing than those who do not develop heart failure after a first myocardial infarction.
The NOTION 2 trial, which recently enrolled a 64-year-old female with Society of Thoracic Surgeon (STS) score 1.2% as its first patient, is comparing the use of TAVI with the use of surgical aortic valve replacement in patients aged ≤75 years at low surgical risk.
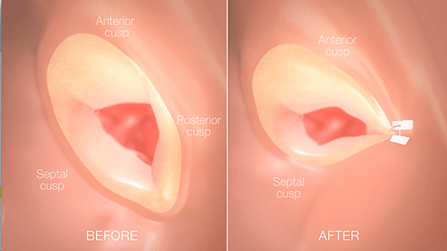
Martina Patané and colleagues found that percutaneous edge-to-edge repair (MitraClip, Abbott Vascular), either as a planned staged treatment or as a bailout therapy, is feasible and effective approach in mitral regurgitation patients who have undergone TAVI.
QT Vascular has received CE mark clearance for the sale and distribution of its Chocolate Heart drug-coated balloon for dilatation of the stenotic portion of coronary arteries for the purpose of improving myocardial perfusion in Europe.
Cardiovascular Systems Inc (CSI) has submitted an application to Japan's Pharmaceuticals and Medical Devices Agency (PMDA) for approval of its Diamondback 360 Coronary Orbital Atherectomy System Micro Crown to treat severely calcified coronary arteries for the facilitation of stent placement.
SynCardia Systems has entered into an asset purchase agreement with an affiliate of Versa Capital Management to acquire substantially all of the company's assets and operations, bringing with it the ability to provide the capital necessary for SynCardia to realise its full potential as the world's first and only FDA, provider of the Total Artificial Heart and drivers.
The Tryton Confirmatory Study, recently published in JACC Cardiovascular Interventions, has confirmed the safety and efficacy of the Tryton Side Branch Stent for the treatment of coronary bifurcation lesions involving large side branches (appropriate for a ≥2.5mm stent).
Corindus Vascular Robotics' CorPath system will be highlighted in several presentations on new therapies and techniques for the treatment of high-risk patients with complex cardiovascular disease at the San Diego Cardiovascular Interventions Course (SDCI; 8–9 July, San Diego, USA).
Five years after receiving the CE mark, Abbott Vascular’s bioresorbable ...
A 64-year old female with severe aortic stenosis and low surgical risk (STS score 1.2%) has been treated at Rigshospitalet in Copenhagen, Denmark, with transcatheter aortic valve implantation (TAVI), becoming the first patient in the NOTION-2 trial.
A study published in the American Journal of Critical Care has found a high prevalence of delirium in a small cohort of critically ill patients treated with therapeutic hypothermia after cardiac arrest.
A study, published in the JAMA: Internal Medicine, indicates that doctors who receive industry-sponsored meals have higher rates of brand-name drug prescriptions than alternative options within the same drug class.
RenalGuard Solutions has been featured in a high-risk coronary chronic total occlusion (CTO) live case presentation at the recent CTO Fundamentals Course, June 3, 2016, VU University Medical Center, Amsterdam, The Netherlands.
BioVentrix has received certification for CE marking its Revivent TC transcatheter ventricular enhancement system.
A simple preoperative echocardiographic measurement of the amount of torsion of the heart predicted outcomes of mitral valve surgery in some heart failure patients, according to a novel study published in JACC: Basic to Translational Science.
QT Vascular, together with its subsidiaries, has receivedFDA 510(k) clearance for the Chocolate XD percutaneous transluminal coronary angioplasty catheter for balloon dilatation of the stenotic portion of coronary artery or bypass graft stenosis for the purpose of improving myocardial perfusion including in-stent restenosis.
A network meta-analysis of early generation, drug-eluting stents, bare metal stents, drug-coated balloons, and balloon angioplasty indicate that sirolimus-eluting stents are associated with the most favourable angiographic and clinical outcomes for lesions in small coronary arteries.
Grant W Reed, Milind T Desai and others report that prior external beam radiation therapy is an independent predictor of both all-cause and cardiovascular mortality in patients undergoing PCI.
HeartFlow has announced that it has launched its next generation of its HeartFlow FFRct system. The result of years of development, the next-generation platform includes major advancements in the process and algorithms HeartFlow uses to calculate fractional flow reserve computed tomography.
Initially designed as a treatment for inoperable patients with aortic st...
The internet age has enabled patients to access a plethora of medical information. However, a potential drawback is that some of this information is inaccurate or misleading, causing unnecessary anxiety or false hope to patients.
The first patients have been treated using CorMatrix Cardiovascular’s Tyke.
T...
The first patient has been enrolled in the FROST study at William Beaumont Hospi...
Positive results of a single-centre, retrospective study using the Neovasc Reduc...
Measuring antibody levels in the blood could be used to detect a person’s he...
St Jude Medical has received an Innovation Award for its HeartMate 3 left ve...
Biotronik’s bioresorbable magnesium scaffold—Magmaris—is now CE mark approve...
The product received US Food and Drug Administration 510(k) clearance in February 2016. It is intended for use in neonates and infants for repair of pericardial structures, as an epicardial covering for damaged or repaired cardiac structures, as a patch material for intracardiac defects, septal defects and annulus repair, suture-line buttressing, and cardiac repair.
The first patient has been enrolled in the FROST study at William Beaumont Hospital, Dearborn, USA. This AtriCure-sponsored trial will evaluate intraoperative cryoanalgesia therapy using cryoablation in conjunction with standard of care (SOC) pain management compared to SOC alone.
The Cardiac & Vascular Institute is the first organisation in the Nort...
Highlights - TAVI with Sapien 3 may be better than surgery for intermediate-risk...
Highlights - TAVI with Sapien 3 may be better than surgery for intermediate-risk...
Highlights - ABSORB III supports previous findings that Absorb is noninferior to...
Highlights - ABSORB III supports previous findings that Absorb is noninferior to...
Highlights - Novel diagnostic strategy may "dramatically reduce need for invasiv...
Highlights - Novel diagnostic strategy may "dramatically reduce need for invasiv...
Highlights: -Sapien XT and CoreValve have comparable mortality at one year -Curr...
Highlights: -Sapien XT and CoreValve have comparable mortality at one year -Curr...
Highlights: -In BEST, surgery is still top option for multivessel disease -Two-y...
Highlights: -In BEST, surgery is still top option for multivessel disease -Two-y...
Highlights: -More data needed for routine use of TAVI in lower risk patients -Fi...
Highlights: -More data needed for routine use of TAVI in lower risk patients -Fi...
Highlights: -European guidelines lower duration of dual antiplatelet therapy to ...
Highlights: -European guidelines lower duration of dual antiplatelet therapy to ...
Highlights: -Staged intervention is right way to treat STEMI patients with multi...
Highlights: -Staged intervention is right way to treat STEMI patients with multi...
Highlights: - Renal denervation comes to a "grinding halt" but studies will cont...
Highlights: - Renal denervation comes to a "grinding halt" but studies will cont...
Highlights: -Major setback for renal denervation -Long-term dual antiplatelet th...
Highlights: -Major setback for renal denervation -Long-term dual antiplatelet th...
Highlights: -Preventative angioplasty in non-infarct arteries benefits STEMI pat...
Highlights: -Preventative angioplasty in non-infarct arteries benefits STEMI pat...
Highlights: -Six to 12 months is the right timeframe for dual antiplatelet thera...
Highlights: -Six to 12 months is the right timeframe for dual antiplatelet thera...
Highlights: Cangrelor significantly reduces ischaemic events compared with clopi...
Highlights: Cangrelor significantly reduces ischaemic events compared with clopi...
Valcare Medical, an Accelmed portfolio company has successfully completed the first phase of its first-in-human (FIH) multicentre clinical trial, taking place in Israel and Europe.
The device is being assessed for its ability to protect the brain from emboli during transcatheter aortic valve implantation (TAVI), minimising the risk of cerebral damage. The study, REFLECT, is a multicentre, phase 2/3, randomised, interventional, single-blind clinical study.
Georg Nickenig and others report in the Journal of the American College of Cardiology that the Mitralign percutaneous annuoplasty system (MPAS, Mitralign) is a feasible and safe treatment for high-risk patients with functional mitral regurgitation.
Highlights:
-TRILOGY-ACS shows no advantage for prasugrel over clopidogrel
...
Highlights:
-TRILOGY-ACS shows no advantage for prasugrel over clopidogrel
...
Highlights: -The future of renal denervation rests on selecting the right patien...
Highlights: -The future of renal denervation rests on selecting the right patien...
Highlights: -TAVI as effective as surgery in high-risk patients at two years -Su...
Highlights:
-iFR vasodilator-free measure of stenosis severity comparable to FF...
Highlights:
-iFR vasodilator-free measure of stenosis severity comparable to FF...
Highlights:
-Lower thrombosis risk with newer generation drug-eluting stents
-...
Highlights:
-FFR-CT technique can cut unnecessary coronary procedures
-"TAVI s...
Highlights:
-PARTNER find TAVI as good as surgery
-Absorb shows positive one-y...
Highlights
- New absorbable stent era begins
- TAVI improves quality of life i...
Highlights
- ESC sees SHIFT in heart failure treatment
- FDA advisory committe...
Highlights
- "Encouraging" TAVI results in high-risk patients
- Bioresorbable ...
Highlights
- NO ACCORDance on role of fibrates
- Cypher versus ENDEAVOR trial ...
Highlights
- Controversy surrounds long-term lipid lowering therapy
- SPIRIT I...
Highlights
- New drugs steal the spotlight at the ESC Congress
- SYNTAX at two...
Highlights
- Drug-eluting stents "warranted" for infarction
- Syntax Score web...
Highlights
- Percutaneous mitral valve repair takes off
- Antiplatelet agent p...
Highlights
- JUPITER: reduced events in patients with low LDL cholesterol, rais...
PinnacleHealth (East Cowes, USA) has enrolled the first patient nationally in a new clinical trial investigating a larger size of the Medtronic CoreValve Evolut R system, the Evolut R 34mm system.
4Tech has announced that its TriCinch device has been used to successfully treat patients suffering from tricuspid regurgitation. The ongoing feasibility study is being conducted at San Raffaele Hospital (Milan, Italy) and in other sites in Italy and Europe.
A study, which was presented at EuroPRevent 2016 (14–15 June, Sophia Antipolis, ...
Biotronik's bioresorbable magnesium scaffold, Magmaris, is now CE mark approved, meaning it is now one of three bioresorbable scaffolds available on the European market (alongside Abbott Vascular's Absorb and Elixir Medical's Desolve.
A network meta-analysis, published in JACC: Cardiovascular Interventions, in...
Vasim Farooq and others report in Circulation Cardiovascular Interventions that the use of a vascular closure device after transfemoral PCI is associated with a minor short-term mortality benefit compared with manual compression.
The Cardiothoracic Surgery Department's Heart Valve Center at NYU Langone Medical Center became the first centre in the world to implant a new heart valve for TAVI in a patient with severe aortic stenosis.
Stentys has announced that it has received the CE mark for the longest version (37mm) of its Xposition S sirolimus-eluting self-apposing stent. The approval of this longer version means that interventional cardiologists will now only need to implant a single self-apposing stent (in relevant longer lesions) rather than a long conventional stent, thus minimising the risk of malapposition and related complications.
Darren Mylotte and his co-authors report that TAVI with transcarotid access is "feasible and is associated with encouraging short- and medium-term clinical outcomes". In this interview, he explains the need for an alternative access approach to transfemoral and reviews the potential benefits of transcarotid access.
Giovanni Amoroso (Department of Cardiology, Onze Lieve Vrouwe Gasthuis, Amsterdam, The Netherlands) is involved in developing the slender transradial interventions concept, which focuses on the maximal miniaturisation of transradial coronary interventions.
The amount a heart "bleeds" following a myocardial infarction can predict the severity of future heart failure, according to research presented at the British Cardiovascular Society (BCS) conference (6–8 June, Manchester, UK). The researchers have now found that this injury is associated with a higher risk of developing heart failure in the months following a heart attack.
Being married could improve your likelihood of surviving a myocardial infarction and is associated with reduced length of hospital stay, according to research presented at the British Cardiovascular Society (BCS) Conference (6–8 June, Manchester, UK).
Last year, the Cardioband mitral reconstruction system (Valtech) was approved in Europe for mitral valve repair. It joins MitraClip (Abbott Vascular) as one of the few options available for patients with mitral regurgitation who are unable to undergo surgery because of a high risk of complications.
Karl-Heinz Kuck has withdrawn his candidacy for president-elect of the European Society of Cardiology (ESC) and also resigned from board of the European Heart Rhythm Association (EHRA). This follows a conviction for fraud.
Last year, the Cardioband mitral reconstruction system (Valtech) was approved in Europe for mitral valve repair. It joins MitraClip (Abbott Vascular) as one of the few options available for patients with mitral regurgitation who are unable to undergo surgery because of a high risk of complications.
While profoundly overworked clinical teams may understandably be reluctant to accept another referral, aggressive, rude or dismissive communication negatively affects both staff and patients. Benjamin C Whitelaw reviews the steps that can be taken to reduce such behaviour.
Although most patients undergoing complex cardiac or vascular interventions tolerate contrast, some will develop contrast-induced acute kidney injury-which is associated with increased mortality. In this commentary, Philippe Garot and Andrew Roy review the aims and objectives of the ongoing STRENGTH trial.
James Blankenship is the 2015-2016 president of SCAI and has been involved with designing and implementing the society's new strategic plan. He talks to Cardiovascular News about the plan and how climbing Kilimanjaro with his three children was a good reminder of what matters most in life.
In collaboration with APACVS, the Getinge Group has launched a new "Harvesting Heroes" campaign. The campaign seeks to recognise healthcare professionals in the field of cardiac surgery as well as celebrate 20 years since the endoscopic vessel harvesting technique was pioneered.
Micro Interventional Devices has received the CE mark for its Permaseal transapical access and closure device. The device allows surgeons to access and close the left-ventricle instantaneously, reliably and without suturing the myocardium.
Women diagnosed with migraines have a slightly increased risk of developing cardiovascular diseases, such as heart attacks and strokes, and are somewhat more likely to die from these conditions than women who do not have migraine, according to findings of a large study published in The BMJ.
Patient enrolment has been completed in The Medicine Company's ORION-1 study of PCSK9si, its investigational RNA interference (RNAi) proprotein convertase subtilisin/kexin type 9 synthesis inhibitor (PCSK9si).
The US Food and Drug Administration (FDA) has finalised its efforts to streamline the "compassionate use" process, used by physicians to access investigational drugs and biologics for patients with limited treatment options.
Occlutech has obtained European CE mark approval for its left atrial appendage, (LAA), occluder. The device is a specifically designed implant for the minimally invasive closure of the LAA, a procedure that minimises the risk of strokes in patients suffering from atrial fibrillation.
Holger Eggebrecht and others report in the European Heart Journal that the lack of an on-site cardiac surgery department should not be a contraindication to centres performing TAVI
Michael Haude (Medical Clinic I, Städtische Kliniken Neuss, Neuss, Germany) and ...
BioVentrix has received US Food and Drug Administration (FDA) investigational device exemption approval to initiate its pivotal ALIVE (American Less Invasive Ventricular Enhancement) clinical trial.
Royal Philips has received CE marking for its cardiac troponin I (cTnI) blood test with the Minicare I-20 handheld device. Minicare cTnI is designed to deliver lab-comparable test results in less than 10 minutes.
A
ccording to Vinod H Thourani (Emory University School of Me...
Sara Ariotti, Marco Valgimigli and others report in JACC: Cardiovascular Interventions that a zotarolimus-eluting stent provides superior safety and efficacy compared with a bare metal stent in patients at high risk of bleeding. They talk to Cardiovascular News about the implications of these findings for the future use of bare metal stents.
According to data presented at EuroPCR (17-20 May, Paris, France), a hybrid sirolimus-eluting stent (Orsiro, Biotronik) is non-inferior to a zotarolimus-eluting (Resolute Intergrity, Medtronic).
The Netherlands presidency of the European Council and representatives of the European Parliament have reached a political agreement on two draft regulations for medical devices. The new regulations are aimed at ensuring that medical devices and in vitro diagnostic medical devices are safe while allowing patients to benefit of innovative health care solutions in a timely manner.
The heart rate may be an indicator of a person's life expectancy. A research team at the Technical University of Munich (Munich, Germany) has to this end analysed an effect which at first seems paradoxical: Minor irregularities in the heartbeat are indicative of a healthy body.
Patients with acute coronary syndromes (ACS) who are at high risk for bleeding have significantly lower rates of target lesion revascularisation and fewer adverse events after undergoing percutaneous coronary intervention (PCI) with a polymer-free biolimus-A (BA9) drug-coated stent than with those receiving a bare metal stent in results from a sub-study of the LEADERS FREE trial reported for the first time in a late-breaker session at EuroPCR 2016.
Keystone Heart has announced that a pooled analysis of three prospective and comparable clinical studies of patients undergoing transcatheter valve implantation (TAVR) in USA and Europe, have shown that TriGuard cerebral protection significantly reduces stroke rate, lowers CNS infarction and reduces total lesion volume, without adversely impacting the safety of the TAVR procedure.
ResMed has announced primary results from a multicentre, randomised controlled phase II trial-CAT-HF-presented at the European Society of Cardiology's 2016 Annual Heart Failure Congress.
Mitralign has raised US$39.8 million to date in a Series E equity round of financing. With the Series E financing raised, the company plans to pursue US and CE regulatory approval for the commercialisation of their Trialign system, in parallel with preparations for European commercial launch of their Mitralign percutaneous annuloplasty system.
Medinol has announced that the BioNIR has met its non-inferiority primary end point of angiographic in-stent late loss at six months in the NIREUS trial. The prospective, multicentre, randomised, non-inferiority pivotal study compared it to Medtronic's Resolute Integrity stent.
A federal jury in Boston, USA, has returned a verdict in favour of Edward Lifesciences' CardiAQ in a lawsuit filed against a former service provider, Neovasc. The jury found that Neovasc breached the non-disclosure agreement between the parties, misappropriated CardiAQ's trade secrets, and breached its duty of honest performance to CardiAQ.
Direct Flow Medical presented three-year results from its prospective, multicentre DISCOVER Trial at the 2016 EuroPCR meeting in Paris.
A session, sponsored by Alvimedica Medical Technologies, discussing treatment options for PCI in diabetic patients, took place at EuroPCR (16-20th May 2016, Paris, France).
First-in-human data presented at EuroPCR 2016 have demonstrated that haemodynamic data from HeartFlow may help predict which coronary plaques have the potential to rupture.
New data from the EWOLUTION registry, presented at EuroPCR 2016, confirms safety of the Boston Scientific left atrial appendage closure system (Watchman). The data are from more than 1,000 patients, from across Europe, who received the device and focus on post-procedural drug regimen, impact of centre experience and peri-device leakage.
According to results of the RESPOND post-market study, the Lotus TAVI device is associated with-a press release reports-excellent safety and efficacy outcomes at 30 days post implantation.
Results from the BIOLUX randomised control trial, which were presented at EuroPCR 2016, indicate that the Pantera Lux drug-coated balloon (Biotronik) is angiographically non-inferior to stenting with the latest-generation drug-eluting stents at six months for the treatment of in-stent restenosis.
Recruitment of patients for iVascular's ANCHOR clinical trial has been completed with 104 patients treated with the sirolimus eluting stent Angiolite. The first interim three-month data have been presented at EuroPCR Congress 2016.
St Jude Medical has announced results from two cardiovascular clinical trials presented at EuroPCR 2016. The studies, which look at how St Jude Medical's fractional flow reserve (FFR) technology impacts patient outcomes in acute coronary syndrome and a comparison of left atrial appendage occlusion (LAAO) therapy to standard medical treatment, were presented during hotline sessions.
Corona Regional Medical Center (Corona, USA) has installed Toshiba's Vantage Titan 1.5 Tesla magnetic resonance imaging system. It has also chosen to feature the Aquilion Prime 160 and Aquilion Prime 40 computed tomography machines, also from Toshiba.
One-year follow-up results from a paediatric feasibility study of Xeltis bioabsorbable cardiovascular pulmonary graft have been presented as late-breaker at the 96th American Association for Thoracic Surgery annual meeting.
St Jude Medical has launched the Trifecta valve with Glide Technology (GT) in the USA. The valve is designed for the treatment of patients diagnosed with unhealthy, damaged or malfunctioning aortic heart valves. It is intended to allow for an enhanced valve delivery method to ease implantation in challenging anatomies and during minimally invasive surgical approaches.
At EuroPCR (17-20 May, Paris, France), a new global study that involves 4,300 patients from 34 countries was announced. The study, according to a press release, is set to shed light into the use of short duration DAPT in patients following stenting procedures, with a particular focus on those with a high bleeding risk.
St Jude Medical has announced CE mark approval and European launch of the PressureWire X Guidewire fractional flow reserve (FFR) Measurement System. Designed to identify the severity of narrowings in the coronary arteries of patients with coronary artery disease (CAD), FFR measurement allows for a more effective assessment of coronary lesions (blockages), resulting in more accurate diagnosis.
Edwards Lifesciences has announced that 30-day data from its European post-approval study of the Sapien 3 transcatheter aortic heart valve demonstrated positive patient outcomes, including the lowest reported mortality and stroke rates seen in the SOURCE family of registries.
Edwards Lifesciences has announced positive clinical trial results on two of its advanced innovations in surgical heart valves for the treatment of people with aortic valve disease. Data from three studies-COMMENCE, TRANSFORM and FOUNDATION-were presented as part of the late-breaking sessions at the American Association for Thoracic Surgery's (AATS) 96th annual meeting.
Corvia Medical has been granted CE mark approval for its InterAtrial shunt device (IASD). The IASD is a transcatheter device designed to treat heart failure with preserved ejection fraction (HFpEF), previously called diastolic heart failure.