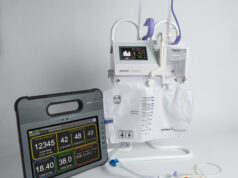
The United States Food & Drug Administration (FDA) has granted 510(k) clearance for ClearFlow’s proprietary FlowGlide active clearance technology system.
The United States Food & Drug Administration (FDA) has granted 510(k) clearance for ClearFlow’s proprietary FlowGlide active clearance technology system.
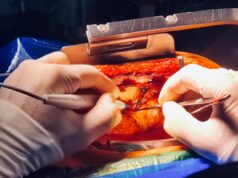
The device is the next generation of ClearFlow’s active clearance technology, designed to prevent or reduces chest drains from occluding with clot, which can lead to retained blood around the heart and lungs.
“This new generation of devices will offer features and benefits never before seen in ClearFlow products. It will also be available in shapes and sizes that previously were not available in our first generation of PleuraFlow devices,” said ClearFlow chief excutive officer Paul Molloy.
“Active clearance of chest tubes has been shown in clinical trials to be superior to passive drainage in preventing RBS,” added cardiothoracic surgeon and ClearFlow co-founder Ed Boyle. “The new design helps resist kinks, and allows positioning of the catheters into harder-to-reach areas in the postoperative chest, effectively expanding the use within the field of cardiothoracic surgery.”