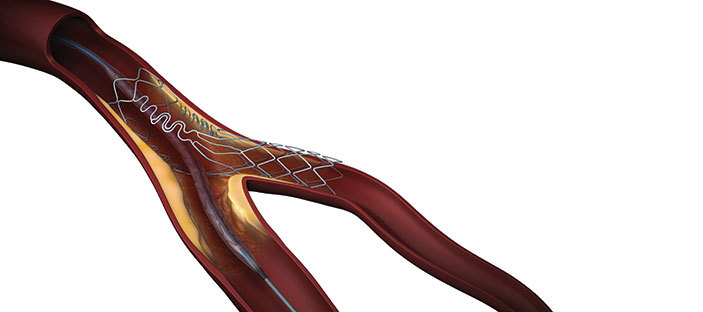
The Tryton Confirmatory Study, recently published in JACC Cardiovascular Interventions, has confirmed the safety and efficacy of the Tryton Side Branch Stent for the treatment of coronary bifurcation lesions involving large side branches (appropriate for a ≥2.5mm stent). In the study, the outcomes of 133 patients who underwent percutaneous coronary intervention (PCI) with the Tryton Side Branch Stent were compared to a performance goal based on performance of the control arm from the Tryton Randomized Controlled Trial (RCT).
A press release reports that the study met its pre-specified primary endpoint (periprocedural myocardial infarction), which was within its non-inferiority margin (primary endpoint: 10.5% + 95% C.I. vs. 17.9%; p=0.01). It adds that the results of the study, and those of the Tryton RCT, have been submitted to the FDA to support the premarket approval application for the Tryton Side Branch Stent.
Principal investigator Martin B Leon (director of the Center for Interventional Vascular Therapy at Columbia University Medical Center, New York-Presbyterian Hospital, New York, USA) says: “In light of the higher procedural success rate, improved acute angiographic results, and higher rate of side branch patency at nine months compared to provisional stenting, the Tryton Confirmatory Study and the Tryton RCT support the use of the dedicated bifurcation Tryton stent.”












