BIBA Publishing
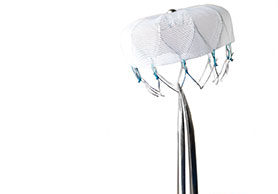
FDA approval for Permaseal transapical access and closure device
Micro Interventional Devices has received FDA market clearance for its Permaseal transapical a...
Oral abstract on DuraGraft to be presented at EACTS
Miguel Haime (VA Boston Healthcare System and Boston Medical Center, Boston, USA) is to present an abstract about Somhulation's DuraGraft during a rapid response session at the 2016 annual meeting of the European Association for Cardio-Thoracic Surgery (EACTS; 1–5 October, Barcelona, Spain).
The American Academy of Neurology says no to routine PFO closure for secondary stroke prevention
In revised recommendations, the American Academy of Neurology (AAN) states that catheter-based closure should not be routinely recommended for people who have had a stroke and also have patent foramen ovale (PFO). The practice advisory, which updates a previous AAN guideline, has been published in Neurology.
US FDA releases draft guidance on real-world evidence and medical device regulation decisions
According to an email from the consumer watchdog, the document is intended to clarify "how the FDA determines that real-world data may be sufficient for use in premarket and postmarket regulatory decisions, without changing the evidentiary standards we use to make those decisions."
ClearFlow launches smaller PleuraFlow active clearance technology for paediatric use
After receiving clearance from the US Food & Drug Administration (FDA) earlier this year for expanded Indications for Use with the company's patented technology, ClearFlow has developed a new model of the PleuraFlow product for the paediatric market.
Providence Health Care adopts HeartFlow FFRct Analysis to assess coronary artery disease
Providence Health Care has become the first centre in Canada to adopt the HeartFlow FFRct Analysis, and also first in the world to use the next generation version of the platform. The HeartFlow FFRct Analysis, which was recently approved by Health Canada, is a non-invasive technology used by clinicians to assess their patients for coronary artery disease.
New website for Xenios
Xenios AG, a developer of lung and heart assist therapies, has combined its novalung, i-cor, and medos websites into www.xenios-ag.com to further advance the Xenios platform. With the new Xenios website, the Heilbronn-based medical device company further advances its lung and heart assist therapy platform.
Study indicates signal for valve degeneration in TAVI patients by eight years
Speaking at EuroPCR (17–20 May, Paris, France), Danny Dvir reported that there is a significant increase in valve degeneration between five and seven years after a transcatheter aortic valve implantation (TAVI) device is implanted.
TAVI durability: A rose by any other name is still a rose
The data that Dvir presented at EuroPCR, as reported by Cardiovascular News, indicate that there is a significant increase in valve degeneration between five and seven years after a transcatheter aortic valve implantation (TAVI) device is implanted.
First patient enrolled in left main study of Xposition S
Stentys has started to enrol patients in its TRUNC trial, which is designed to evaluate the long-term safety and efficacy of the Xposition S stent in the treatment of unprotected left main coronary artery disease.
Safety and efficacy of Carillon mitral contour system confirmed
According to a study published in Open Heart, treatment with the Carillon device (Cardiac Dimensions) significantly reduces annular dimensions and improved mitral regurgitation, heart failure symptoms, and functional capacity in patients with functional mitral regurgitation.
Robotic-assisted PCI is safe and feasible for complex procedures
Results from the CORA-PCI (Complex robotically assisted percutaneous coronary intervention) indicate that robotic-assisted percutaneous coronary intervention (PCI), using the CorPath system (Corindus Vascular Robotics), is a safe and feasible approach to managing patients with complex lesions.
Moderate risk of upper gastrointestinal bleeding after TAVI
A large cohort study, published in Catheterization and Cardiovascular Interventions, indicates that TAVI is associated with an overall 2% risk of upper gastrointestinal bleeding.
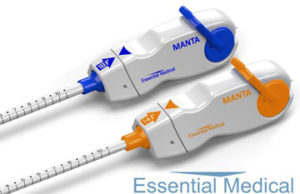
Manta large bore vascular closure device now approved in Europe
Essential Medical has received CE mark approval for Manta; its large bore vascular closure device. The device is a novel vascular closure device designed to close punctures ranging from 10F to 24F at femoral arterial access sites after cardiac catheterisation procedures such as TAVI.
AHA warn that some over-the-counter drugs may cause or worsen heart failure
Commonly used medications and nutritional supplements may cause or worsen heart failure, according to the first scientific statement from the American Heart Association (AHA) to provide guidance on avoiding drug-drug or drug-condition interactions for people with heart failure.
Health Canada approves Abbott Absorb stent
Health Canada has approved Abbott's Absorb bioresorbable heart stent, making the device commercially available to treat people in Canada with coronary artery disease.
Enrolment completed in US early feasibility study on tricuspid repair
Mitralign has announced that enrolment in the first phrase of its SCOUT study, which is evaluating percutaneous tricuspid repair with the Trialign system in patients with functional tricuspid regurgitation, has been completed.
“Strikingly higher” survival rates with Sapien 3 at one year compared with older devices
Overall one-year survival was over 85% for high-risk or inoperable patients who underwent transcatheter aortic valve implantation (TAVI) with Sapien 3, according to a study published in Circulation.
Heart failure after first myocardial infarction linked to cancer
A study published in the Journal of the American College of Cardiology (JACC) indicates that patients who develop heart failure after their first myocardial infarction have a greater risk of developing than those who do not develop heart failure after a first myocardial infarction.
Essential Medical to begin US clinical trial of its large bore vascular closure device
Essential Medical has received IDE approval from the FDA to begin the US clinical trial of its large bore vascular closure device (Manta). The study will evaluate the safety and efficacy of vascular access closure using Manta for femoral arterial access site.
Sunshine Heart to focus on neuromodulation for heart failure
Sunshine Hearth has updated its clinical strategy, which it says could benefit an under-served population of patients with Class III heart failure and other related conditions. The company is moving forward with a therapeutic strategy focused on neuromodulation rather than counterpulsation.
First single monthly injection of a PCSK9 Inhibitor approved in the USA
Amgen has received FDA approval for its Repatha (evolocumab) Pushtronex system (on-body infusor with prefilled cartridge), a new, monthly single-dose administration option. The Pushtronex system is a hands-free device designed to provide 420mg of Repatha in a single dose.
Exploring the borders of TAVI
The NOTION 2 trial, which recently enrolled a 64-year-old female with Society of Thoracic Surgeon (STS) score 1.2% as its first patient, is comparing the use of TAVI with the use of surgical aortic valve replacement in patients aged ≤75 years at low surgical risk.
Combined percutaneous treatment of aortic stenosis and mitral regurgitation is feasible
Martina Patané and colleagues found that percutaneous edge-to-edge repair (MitraClip, Abbott Vascular), either as a planned staged treatment or as a bailout therapy, is feasible and effective approach in mitral regurgitation patients who have undergone TAVI.
Cardiovascular Systems submits Diamondback 360 Coronary Orbital Atherectomy System Micro Crown for approval in Japan
Cardiovascular Systems Inc (CSI) has submitted an application to Japan's Pharmaceuticals and Medical Devices Agency (PMDA) for approval of its Diamondback 360 Coronary Orbital Atherectomy System Micro Crown to treat severely calcified coronary arteries for the facilitation of stent placement.
CE mark for QT Vascular’s Chocolate Heart drug-coated balloon
QT Vascular has received CE mark clearance for the sale and distribution of its Chocolate Heart drug-coated balloon for dilatation of the stenotic portion of coronary arteries for the purpose of improving myocardial perfusion in Europe.
CorPath to be featured at San Diego Cardiovascular Interventions Course
Corindus Vascular Robotics' CorPath system will be highlighted in several presentations on new therapies and techniques for the treatment of high-risk patients with complex cardiovascular disease at the San Diego Cardiovascular Interventions Course (SDCI; 8–9 July, San Diego, USA).
Study confirms safety of using Tryton stent to treat coronary bifurcation lesions involving large side branches
The Tryton Confirmatory Study, recently published in JACC Cardiovascular Interventions, has confirmed the safety and efficacy of the Tryton Side Branch Stent for the treatment of coronary bifurcation lesions involving large side branches (appropriate for a ≥2.5mm stent).
Versa Capital Management to acquire SynCardia Systems
SynCardia Systems has entered into an asset purchase agreement with an affiliate of Versa Capital Management to acquire substantially all of the company's assets and operations, bringing with it the ability to provide the capital necessary for SynCardia to realise its full potential as the world's first and only FDA, provider of the Total Artificial Heart and drivers.
High prevalence of delirium found in cardiac arrest patients with therapeutic hypothermia
A study published in the American Journal of Critical Care has found a high prevalence of delirium in a small cohort of critically ill patients treated with therapeutic hypothermia after cardiac arrest.
First patient treated in NOTION-2 trial of TAVI in younger patients
A 64-year old female with severe aortic stenosis and low surgical risk (STS score 1.2%) has been treated at Rigshospitalet in Copenhagen, Denmark, with transcatheter aortic valve implantation (TAVI), becoming the first patient in the NOTION-2 trial.
Industry-sponsored meals linked to increased rate of prescribing brand-name drugs
A study, published in the JAMA: Internal Medicine, indicates that doctors who receive industry-sponsored meals have higher rates of brand-name drug prescriptions than alternative options within the same drug class.
FDA clears QT Vascular Chocolate XD balloon
QT Vascular, together with its subsidiaries, has receivedFDA 510(k) clearance for the Chocolate XD percutaneous transluminal coronary angioplasty catheter for balloon dilatation of the stenotic portion of coronary artery or bypass graft stenosis for the purpose of improving myocardial perfusion including in-stent restenosis.
Twisting of the heart may predict mitral valve surgery outcomes
A simple preoperative echocardiographic measurement of the amount of torsion of the heart predicted outcomes of mitral valve surgery in some heart failure patients, according to a novel study published in JACC: Basic to Translational Science.
Bioventrix receives CE marking for Revivent TC system
BioVentrix has received certification for CE marking its Revivent TC transcatheter ventricular enhancement system.
RenalGuard features in high-risk live case at CTO Fundamentals Course
RenalGuard Solutions has been featured in a high-risk coronary chronic total occlusion (CTO) live case presentation at the recent CTO Fundamentals Course, June 3, 2016, VU University Medical Center, Amsterdam, The Netherlands.
Prior radiation therapy is associated with increased risk of mortality in PCI patients
Grant W Reed, Milind T Desai and others report that prior external beam radiation therapy is an independent predictor of both all-cause and cardiovascular mortality in patients undergoing PCI.
Trials on newer generation drug-eluting stents in small coronary vessels needed
A network meta-analysis of early generation, drug-eluting stents, bare metal stents, drug-coated balloons, and balloon angioplasty indicate that sirolimus-eluting stents are associated with the most favourable angiographic and clinical outcomes for lesions in small coronary arteries.
Next-generation of HeartFlow launched
HeartFlow has announced that it has launched its next generation of its HeartFlow FFRct system. The result of years of development, the next-generation platform includes major advancements in the process and algorithms HeartFlow uses to calculate fractional flow reserve computed tomography.
Online medical information: A blessing or a curse?
The internet age has enabled patients to access a plethora of medical information. However, a potential drawback is that some of this information is inaccurate or misleading, causing unnecessary anxiety or false hope to patients.
Cardiac & Vascular Institute becomes first North Central Florida cardiologist group to offer Watchman LAAC implant
The Cardiac & Vascular Institute is the first organisation in the North Central Florida US...
Percutaneous direct annuloplasty is “safe and feasible” for functional mitral regurgitation
Georg Nickenig and others report in the Journal of the American College of Cardiology that the Mitralign percutaneous annuoplasty system (MPAS, Mitralign) is a feasible and safe treatment for high-risk patients with functional mitral regurgitation.
First patient enrolled in Keystone Heart Triguard study
The device is being assessed for its ability to protect the brain from emboli during transcatheter aortic valve implantation (TAVI), minimising the risk of cerebral damage. The study, REFLECT, is a multicentre, phase 2/3, randomised, interventional, single-blind clinical study.
Valcare completes first phase of first-in-human Amend trial
Valcare Medical, an Accelmed portfolio company has successfully completed the first phase of its first-in-human (FIH) multicentre clinical trial, taking place in Israel and Europe.
Transcatheter tricuspid valve repair successfully achieved in feasibility study of 4Tech TriCinch
4Tech has announced that its TriCinch device has been used to successfully treat patients suffering from tricuspid regurgitation. The ongoing feasibility study is being conducted at San Raffaele Hospital (Milan, Italy) and in other sites in Italy and Europe.
Investigational trial into Medtronic CoreValve Evolut R 34mm enrols first patient
PinnacleHealth (East Cowes, USA) has enrolled the first patient nationally in a new clinical trial investigating a larger size of the Medtronic CoreValve Evolut R system, the Evolut R 34mm system.
Biotronik’s scaffold is now approved for use in Europe
Biotronik's bioresorbable magnesium scaffold, Magmaris, is now CE mark approved, meaning it is now one of three bioresorbable scaffolds available on the European market (alongside Abbott Vascular's Absorb and Elixir Medical's Desolve.
Minor short-term mortality benefit with vascular closure devices
Vasim Farooq and others report in Circulation Cardiovascular Interventions that the use of a vascular closure device after transfemoral PCI is associated with a minor short-term mortality benefit compared with manual compression.
Go “slender” to reduce the rate of radial artery occlusions
Giovanni Amoroso (Department of Cardiology, Onze Lieve Vrouwe Gasthuis, Amsterdam, The Netherlands) is involved in developing the slender transradial interventions concept, which focuses on the maximal miniaturisation of transradial coronary interventions.
“Encouraging” results for transcarotid TAVI
Darren Mylotte and his co-authors report that TAVI with transcarotid access is "feasible and is associated with encouraging short- and medium-term clinical outcomes". In this interview, he explains the need for an alternative access approach to transfemoral and reviews the potential benefits of transcarotid access.
CE mark for longest version of Xposition S sirolimus-eluting stent
Stentys has announced that it has received the CE mark for the longest version (37mm) of its Xposition S sirolimus-eluting self-apposing stent. The approval of this longer version means that interventional cardiologists will now only need to implant a single self-apposing stent (in relevant longer lesions) rather than a long conventional stent, thus minimising the risk of malapposition and related complications.
NYU Langone becomes first centre in world to use Evolut PRO TAVI system
The Cardiothoracic Surgery Department's Heart Valve Center at NYU Langone Medical Center became the first centre in the world to implant a new heart valve for TAVI in a patient with severe aortic stenosis.
James Blankenship
James Blankenship is the 2015-2016 president of SCAI and has been involved with designing and implementing the society's new strategic plan. He talks to Cardiovascular News about the plan and how climbing Kilimanjaro with his three children was a good reminder of what matters most in life.
The risk of acute kidney injury during complex cardiac interventions: Rationale of the STRENGTH study
Although most patients undergoing complex cardiac or vascular interventions tolerate contrast, some will develop contrast-induced acute kidney injury-which is associated with increased mortality. In this commentary, Philippe Garot and Andrew Roy review the aims and objectives of the ongoing STRENGTH trial.
Reducing aggressive and rude communication helps both doctors and patients
While profoundly overworked clinical teams may understandably be reluctant to accept another referral, aggressive, rude or dismissive communication negatively affects both staff and patients. Benjamin C Whitelaw reviews the steps that can be taken to reduce such behaviour.
A new percutaneous approach for managing mitral valve regurgitation
Last year, the Cardioband mitral reconstruction system (Valtech) was approved in Europe for mitral valve repair. It joins MitraClip (Abbott Vascular) as one of the few options available for patients with mitral regurgitation who are unable to undergo surgery because of a high risk of complications.
Karl-Heinz Kuck steps down from the ESC and EHRA after fraud conviction
Karl-Heinz Kuck has withdrawn his candidacy for president-elect of the European Society of Cardiology (ESC) and also resigned from board of the European Heart Rhythm Association (EHRA). This follows a conviction for fraud.
A new percutaneous approach for managing mitral valve regurgitation
Last year, the Cardioband mitral reconstruction system (Valtech) was approved in Europe for mitral valve repair. It joins MitraClip (Abbott Vascular) as one of the few options available for patients with mitral regurgitation who are unable to undergo surgery because of a high risk of complications.
Marriage is good for the heart
Being married could improve your likelihood of surviving a myocardial infarction and is associated with reduced length of hospital stay, according to research presented at the British Cardiovascular Society (BCS) Conference (6–8 June, Manchester, UK).
Heart bleeds associated with severity of heart failure
The amount a heart "bleeds" following a myocardial infarction can predict the severity of future heart failure, according to research presented at the British Cardiovascular Society (BCS) conference (6–8 June, Manchester, UK). The researchers have now found that this injury is associated with a higher risk of developing heart failure in the months following a heart attack.
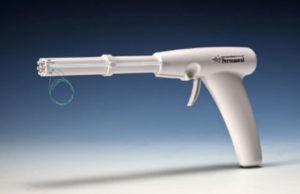
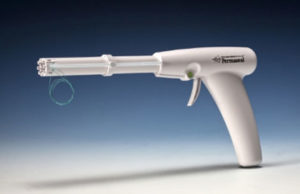
Permaseal transapical access and closure device now approved in Europe
Micro Interventional Devices has received the CE mark for its Permaseal transapical access and closure device. The device allows surgeons to access and close the left-ventricle instantaneously, reliably and without suturing the myocardium.
New “harvesting heroes” campaign to recognise work of healthcare professionals working in cardiac surgery
In collaboration with APACVS, the Getinge Group has launched a new "Harvesting Heroes" campaign. The campaign seeks to recognise healthcare professionals in the field of cardiac surgery as well as celebrate 20 years since the endoscopic vessel harvesting technique was pioneered.
FDA streamlines “compassionate use” application process
The US Food and Drug Administration (FDA) has finalised its efforts to streamline the "compassionate use" process, used by physicians to access investigational drugs and biologics for patients with limited treatment options.
The Medicines Company completes patient enrolment for ORION-1 study of PCSK9si
Patient enrolment has been completed in The Medicine Company's ORION-1 study of PCSK9si, its investigational RNA interference (RNAi) proprotein convertase subtilisin/kexin type 9 synthesis inhibitor (PCSK9si).
Study shows women with migraines to be at higher risk of cardiovascular disease and mortality
Women diagnosed with migraines have a slightly increased risk of developing cardiovascular diseases, such as heart attacks and strokes, and are somewhat more likely to die from these conditions than women who do not have migraine, according to findings of a large study published in The BMJ.
Lack of an on-site cardiac surgery department should not be a barrier to TAVI
Holger Eggebrecht and others report in the European Heart Journal that the lack of an on-site cardiac surgery department should not be a contraindication to centres performing TAVI
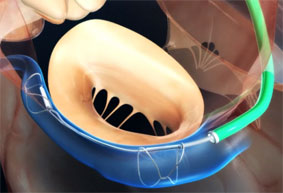
CE mark granted to Occlutech left atrial appendage occluder
Occlutech has obtained European CE mark approval for its left atrial appendage, (LAA), occluder. The device is a specifically designed implant for the minimally invasive closure of the LAA, a procedure that minimises the risk of strokes in patients suffering from atrial fibrillation.
Philips Minicare I-20 handheld devices receives CE mark
Royal Philips has received CE marking for its cardiac troponin I (cTnI) blood test with the Minicare I-20 handheld device. Minicare cTnI is designed to deliver lab-comparable test results in less than 10 minutes.
BioVentrix receives FDA investigative device exemption for ALIVE trial of Revivent
BioVentrix has received US Food and Drug Administration (FDA) investigational device exemption approval to initiate its pivotal ALIVE (American Less Invasive Ventricular Enhancement) clinical trial.
European Union agrees new rules for approving medical devices and in vitro diagnostic medical devices
The Netherlands presidency of the European Council and representatives of the European Parliament have reached a political agreement on two draft regulations for medical devices. The new regulations are aimed at ensuring that medical devices and in vitro diagnostic medical devices are safe while allowing patients to benefit of innovative health care solutions in a timely manner.
Trial shows Orsiro is non-inferior to Resolute Integrity
According to data presented at EuroPCR (17-20 May, Paris, France), a hybrid sirolimus-eluting stent (Orsiro, Biotronik) is non-inferior to a zotarolimus-eluting (Resolute Intergrity, Medtronic).
More data in support of newer generation stents for high bleeding risk patients
Sara Ariotti, Marco Valgimigli and others report in JACC: Cardiovascular Interventions that a zotarolimus-eluting stent provides superior safety and efficacy compared with a bare metal stent in patients at high risk of bleeding. They talk to Cardiovascular News about the implications of these findings for the future use of bare metal stents.
Polymer-free coronary stent more effective and safer than bare metal stent in ACS patients
Patients with acute coronary syndromes (ACS) who are at high risk for bleeding have significantly lower rates of target lesion revascularisation and fewer adverse events after undergoing percutaneous coronary intervention (PCI) with a polymer-free biolimus-A (BA9) drug-coated stent than with those receiving a bare metal stent in results from a sub-study of the LEADERS FREE trial reported for the first time in a late-breaker session at EuroPCR 2016.
New procedure uses heart rate to estimate life expectancy of infarct patients
The heart rate may be an indicator of a person's life expectancy. A research team at the Technical University of Munich (Munich, Germany) has to this end analysed an effect which at first seems paradoxical: Minor irregularities in the heartbeat are indicative of a healthy body.
Direct Flow Medical reports DISCOVER trial three-year results
Direct Flow Medical presented three-year results from its prospective, multicentre DISCOVER Trial at the 2016 EuroPCR meeting in Paris.
Jury favours CardiAQ in dispute with Neovasc
A federal jury in Boston, USA, has returned a verdict in favour of Edward Lifesciences' CardiAQ in a lawsuit filed against a former service provider, Neovasc. The jury found that Neovasc breached the non-disclosure agreement between the parties, misappropriated CardiAQ's trade secrets, and breached its duty of honest performance to CardiAQ.
Medinol announces results for NIREUS trial of BioNIR stent
Medinol has announced that the BioNIR has met its non-inferiority primary end point of angiographic in-stent late loss at six months in the NIREUS trial. The prospective, multicentre, randomised, non-inferiority pivotal study compared it to Medtronic's Resolute Integrity stent.
Mitralign raises almost US$40 million in equity financing
Mitralign has raised US$39.8 million to date in a Series E equity round of financing. With the Series E financing raised, the company plans to pursue US and CE regulatory approval for the commercialisation of their Trialign system, in parallel with preparations for European commercial launch of their Mitralign percutaneous annuloplasty system.
ResMed announces primary results for CAT-HF sleep apnoea trial
ResMed has announced primary results from a multicentre, randomised controlled phase II trial-CAT-HF-presented at the European Society of Cardiology's 2016 Annual Heart Failure Congress.
Pooled trial results show positive outcomes for Keystone Heart’s TriGuard
Keystone Heart has announced that a pooled analysis of three prospective and comparable clinical studies of patients undergoing transcatheter valve implantation (TAVR) in USA and Europe, have shown that TriGuard cerebral protection significantly reduces stroke rate, lowers CNS infarction and reduces total lesion volume, without adversely impacting the safety of the TAVR procedure.
HeartFlow haemodynamic data may help predict coronary plaque rupture potential
First-in-human data presented at EuroPCR 2016 have demonstrated that haemodynamic data from HeartFlow may help predict which coronary plaques have the potential to rupture.
Alvimedica TNT session presented at EuroPCR
A session, sponsored by Alvimedica Medical Technologies, discussing treatment options for PCI in diabetic patients, took place at EuroPCR (16-20th May 2016, Paris, France).
St Jude Medical launches new Trifecta GT tissue valve in the USA
St Jude Medical has launched the Trifecta valve with Glide Technology (GT) in the USA. The valve is designed for the treatment of patients diagnosed with unhealthy, damaged or malfunctioning aortic heart valves. It is intended to allow for an enhanced valve delivery method to ease implantation in challenging anatomies and during minimally invasive surgical approaches.
One-year paediatric feasibility study results published for Xeltis bioabsorbable graft
One-year follow-up results from a paediatric feasibility study of Xeltis bioabsorbable cardiovascular pulmonary graft have been presented as late-breaker at the 96th American Association for Thoracic Surgery annual meeting.
Corona Regional Medical Center installs Toshiba’s Vantage Titan and Aquilon Prime systems
Corona Regional Medical Center (Corona, USA) has installed Toshiba's Vantage Titan 1.5 Tesla magnetic resonance imaging system. It has also chosen to feature the Aquilion Prime 160 and Aquilion Prime 40 computed tomography machines, also from Toshiba.
Study suggests major clinical benefit for left atrial appendage occlusion
St Jude Medical has announced results from two cardiovascular clinical trials presented at EuroPCR 2016. The studies, which look at how St Jude Medical's fractional flow reserve (FFR) technology impacts patient outcomes in acute coronary syndrome and a comparison of left atrial appendage occlusion (LAAO) therapy to standard medical treatment, were presented during hotline sessions.
iVascular completes recruitment of patients for ANCHOR clinical trial of Angiolite
Recruitment of patients for iVascular's ANCHOR clinical trial has been completed with 104 patients treated with the sirolimus eluting stent Angiolite. The first interim three-month data have been presented at EuroPCR Congress 2016.
Drug-coated balloon is viable alternative to drug-eluting stent
Results from the BIOLUX randomised control trial, which were presented at EuroPCR 2016, indicate that the Pantera Lux drug-coated balloon (Biotronik) is angiographically non-inferior to stenting with the latest-generation drug-eluting stents at six months for the treatment of in-stent restenosis.
Post-market study show “excellent” outcomes for Lotus TAVI valve
According to results of the RESPOND post-market study, the Lotus TAVI device is associated with-a press release reports-excellent safety and efficacy outcomes at 30 days post implantation.
Three-month data for Watchman show high success rate
New data from the EWOLUTION registry, presented at EuroPCR 2016, confirms safety of the Boston Scientific left atrial appendage closure system (Watchman). The data are from more than 1,000 patients, from across Europe, who received the device and focus on post-procedural drug regimen, impact of centre experience and peri-device leakage.
Edwards’ surgical heart valve innovations demonstrate positive patient benefits
Edwards Lifesciences has announced positive clinical trial results on two of its advanced innovations in surgical heart valves for the treatment of people with aortic valve disease. Data from three studies-COMMENCE, TRANSFORM and FOUNDATION-were presented as part of the late-breaking sessions at the American Association for Thoracic Surgery's (AATS) 96th annual meeting.
Sapien 3 demonstrates positive patient outcomes at 30 days in European real-world experience
Edwards Lifesciences has announced that 30-day data from its European post-approval study of the Sapien 3 transcatheter aortic heart valve demonstrated positive patient outcomes, including the lowest reported mortality and stroke rates seen in the SOURCE family of registries.
St Jude Medical launches PressureWire X Guidewire in Europe
St Jude Medical has announced CE mark approval and European launch of the PressureWire X Guidewire fractional flow reserve (FFR) Measurement System. Designed to identify the severity of narrowings in the coronary arteries of patients with coronary artery disease (CAD), FFR measurement allows for a more effective assessment of coronary lesions (blockages), resulting in more accurate diagnosis.
Major new study to investigate abbreviated DAPT in high bleeding risk patients
At EuroPCR (17-20 May, Paris, France), a new global study that involves 4,300 patients from 34 countries was announced. The study, according to a press release, is set to shed light into the use of short duration DAPT in patients following stenting procedures, with a particular focus on those with a high bleeding risk.
Cordis to return to drug-eluting stent market following Biosensors agreement
Cardinal Health has entered into a distribution agreement with Biosensors that enables Cordis, Cardinal Health's interventional vascular business, to sell Biosensors' coronary stent portfolio.
Corvia Medical InterAtrial shunt device receives CE mark
Corvia Medical has been granted CE mark approval for its InterAtrial shunt device (IASD). The IASD is a transcatheter device designed to treat heart failure with preserved ejection fraction (HFpEF), previously called diastolic heart failure.
Young female acute coronary syndrome patients have significantly greater comorbidities than their male counterparts
A study indicates that women aged less than 55 years with acute coronary syndrome undergoing percutaneous coronary intervention (PCI) have significantly greater comorbidities and worse outcomes than their male counterparts.
Study to directly compare Orsiro with Xience in STEMI patients
The first patient has been enrolled in Biotronik's new BIOSTEMI trial, which is evaluating the safety and efficacy of the Orsiro hybrid drug-eluting stent, compared with Xience Xpedition (Abbott Vascular), in patients with STEMI.
Admedus to attend upcoming US heart conferences
Admedus will be attending the American Association for Thoracic Surgery (AATS) Aortic Symposium 2016 May 12-13 in New York City, USA and the 96th AATS Annual Meeting taking place from 14-18 May in Baltimore, USA.
Medicure files application for new tirofiban hydrochloride product format
Medicure has submitted an application to the US Food and Drug Administration (FDA) for the introduction of a new "bolus vial" product format for tirofiban hydrochloride monohydrate (HCl), trading under the name Aggrastat.
Device reduces volume of radiographic dye in patients at risk of developing acute kidney injury
In the largest study of its kind, the Avert device from Osprey Medical has been found to significantly reduce the volume of radiographic dye without decreasing image quality in patients who are at risk of developing acute kidney injury after undergoing a coronary angiography or percutaneous coronary intervention.
No gender-based outcomes variation for two anticoagulants in TAVR patients
A study on the impact of using different anticoagulation medications on men and women who have undergone a transcatheter aortic valve replacement (TAVR) has found no difference in early vascular complications or mortality.
NICE recommends evolocumab for some patients at high risk of cardiovascular events
NICE has published a recommendation supporting the use of evolocumab alone or in combination with other cholesterol-lowering therapies, for several types of patients at particularly high risk of cardiovascular events with persistently high cholesterol.
Optical coherence tomography helps cardiologist predict significant side branch ostium stenosis
In a new study using optical coherence tomography (OCT), researchers have found that maximum lipid arc and the presence of lipid plaque contralateral to the side branch (SB) ostium before stenting may contribute to significant side branch ostium stenosis (SBOS) after stenting.
Robotically-assisted percutaneous coronary intervention feasible in complex cases
A first-of-its kind study using robotic technology to remotely control coronary guidewires and stents reported on the feasibility of performing percutaneous coronary intervention (PCI) on patients with complex coronary lesions. Similar clinical outcomes compared to the PCI procedure performed manually were reported.
Exploring the role of P2Y12 inhibitor monotherapy after dual antiplatelet therapy
According to Usman Baber, TWILIGHT is a unique and innovative study in that the experimental intervention is to withdraw rather than add to existing background pharmacotherapy. In this commentary, he explores the aims and goals of the study.
First central Pennsylvania, USA, patients receive new aortic valve reconstruction procedure
Three PinnacleHealth patients have undergone a new procedure for aortic valve reconstruction, using the patients’ own heart tissue (pericardium) to create the new valves.
Konica Minolta release updated Sonimage HS1 compact ultrasound
Konica Minolta have introduced a new version of the Sonimage HS1 compact ultrasound system, which is designed to enable improved image quality.
Patient enrolment completed in REDUCE trial
Enrolment in the REDUCE trial-a physician-initiated, prospective, multicentre, randomised study, designed to evaluate the potential for shorter-term dual antiplatelet therapy (DAPT) in acute coronary syndrome-has reached completion, according to an OrbusNeich press release.
Abbott to acquire St Jude Medical
Abbott is set to acquire St Jude Medical, expanding its portfolio to cover cardiovascular markets such as atrial fibrillation, structural heart and heart failure as well as neuromodulation. The combined company will thus produce devices across cardiovascular, diabetes, vision and neuromodulation markets.
Enrolment completed in BIOFLOW-V clinical study or Orsiro
Biotronik has announced that enrolment in its BIOFLOW-V clinical study has been completed. The company reports that 1,334 patients have been enrolled at 91 sites in the US, Canada, Europe, Israel, and the Asia Pacific region in under a year.
Transcatheter technologies sells its technology portfolio to Venus MedTech
Transcatheter Technologies GmbH, a medical device company that has developed a full range of transcatheter valve implantation systems for catheter-based heart valve therapy, has sold its technology portfolio to Venus Medtech.
Study finds reasons for hospital-level variations in bleeding post-angioplasty remain unclear
The use of bleeding avoidance strategies has only a modest effect on the variation in bleeding rates post-angioplasty among hospitals performing this procedure, leaving about 70% of the causes for this variation unexplained, according to a study published in JACC: Cardiovascular Interventions.
Scientists report on novel method for extending the life of implantable devices in situ
In a paper published in Nature Communications, investigators from Harvard report on a novel biochemical method that enables the rapid and repeated regeneration of selected molecular constituents in situ after device implantation, which has the potential to substantially extend the lifetime of bioactive films.
Toshiba’s Aquilion Lightning CT with more powerful generator receives FDA clearance
Toshiba America Medical Systems'sAquilionTM Lightning has been cleared by the US Food and Drug Administration (FDA) with a more powerful 50-kW generator.
Admedus expands distribution agreement with Coroneo
Admedus has expanded its distribution partnership with Coroneo to sell its Extra-Aortic Annuloplasty Ring and related products in Australia and New Zealand. According to a press release, Admedus anticipates gaining marketing approval for this unique aortic implant in the second half of 2016.
French hospital celebrates 30 years of implanting artificial hearts
La Piti̩-Salp̻tri̬re Hospital (Paris, France), the world's leading artificial heart centre, is celebrating the 30th anniversary of the first Total Artificial Heart implantation. The centre has now performed 249 implants of the SynCardia Total Artificial Heart and has implanted more of the devices than any other medical centre in the world.
Boston Scientific voluntarily recalls Fetch 2 aspiration catheter
Boston Scientific has initiated a global, voluntary recall of all models of its Fetch 2 aspiration catheter. A press release reports that the catheters were recalled on 22 March 2016 because of complaints of shaft breakage.
Impella can now be used in USA for cardiogenic shock after myocardial infarction
Abiomed has received FDA pre-market approval for its Impella 2.5, Impella CP, Impella 5.0 and Impella LD heart pumps for the treatment of ongoing cardiogenic shock. This latest approval adds to the prior FDA indication of Impella 2.5 for high risk percutaneous coronary intervention (PCI), or Protected PCI.
Boston Scientific is suspending sales of Watchman FLX in Europe
According to a Reuters news report, Boston Scientific is temporarily suspending sales of Watchman FLX-the next-generation system of its left atrial appendage closure device, Watchman.
St Jude Medical launches Trifecta surgical valve in Europe
A press release reports that the new Trifecta valve with glide technology provides enhanced valve delivery designed to improve implantation during both minimally invasive and conventional valve replacement procedures.
4Tech appoints Paul Cornelison as Global Vice President of Regulatory Affairs, Quality Assurance & Clinical Affairs
4Tech, which is developing a transcatheter device (TriCinch) for repair of the tricuspid valve, has announced that it has appointed Paul Cornelison, as Vice President of Regulatory Affairs, Quality Assurance & Clinical Affairs.
Deferred stenting in STEMI patients does not improve outcomes
A study indicates that delaying stenting-compared with conventional PCI-in patients with STEMI does not reduce the risk of death, heart failure, myocardial infarction, or repeat revascularisation.
New sub-analyses show benefit of long-term use of ticagrelor for high-risk myocardial infarction patients
AstraZeneca has announced results of two separate sub-analyses of PEGASUS-TIMI 54, which investigated the long-term use of ticagrelor (Brilinta) tablets in patients with a history of myocardial infarction and at least one additional risk factor for thrombotic cardiovascular events at three years.
Early data show good rapid healing for Medtronic’s investigational drug-filled stent
One-month, follow-up patient cohort data from the Revelution trial of Medtronic's novel drug-filled stent indicate that device is associated with rapid vessel healing without inflammation, as assessed by OCT.
One-year data for FFRCT support previous findings
According to one-year data, FFRCT (HeartFlow) significantly reduces the need for invasive procedures to diagnose patients suspected of having coronary artery disease and also leads to a sustained reduction in the cost of care.
Positive early feasibility data for pulmonary valve restoration with transcatheter pulmonary valve
Clinical data, from an early feasibility study, indicate that Medtronic's Harmony transcatheter pulmonary calve is associated with positive initial outcomes at six-months in patients with an indication for pulmonary valve restoration.
More than 5,000 patients enrolled in iFR outcome trials
More than 5,000 patients have been enrolled in three prospective clinical studies to assess the safety of deferring cardiovascular interventions iFR pressure measurement technology compared with FFR measurements.
CoreValve still better than surgery at three years in high-risk patients
TAVI with CoreValve (Medtronic) continues to be associated with significantly better outcomes than surgery at three years in high-risk patients. Previously published data for the device have indicated that it has better outcomes at one and two years after the index procedure.
The role of sutureless and rapid-deployment valves
Antonio Miceli explores the data for sutureless and rapid-deployment surgical valves for the management of patients with severe aortic stenosis. He also reviews the place of these new devices alongside traditional surgical valves and TAVI devices.
Toshiba to showcase Aquilion ONE family technology at the 2016 ACC meeting
Toshiba will showcase its Aquilion ONE family of computed tomography (CT) systems at this year's American College of Cardiology (ACC) annual meeting in Chicago, USA, April 2-4, 2016. The systems are designed to improve workflow and make TAVR planning more efficient.
First patients enrolled in Medtronic CoreValve Evolut R trial in low-risk aortic stenosis patients
The first patients have been enrolled in the expanded indication trial for the CoreValve Evolut R next-generation, recapturable, self-expanding transcatheter aortic valve replacement (TAVR) system from Medtronic. This clinical trial will include 1,200 patients with severe aortic stenosis who have a less than 3% risk of operative mortality.
Two new publications report on use of PleuraFlow technology
Cardiac surgeons at the Montreal Heart Institute have published a case report featured in the Annals of Thoracic Surgery, shortly after the publication of a new study in the Journal of Thoracic and Cardiovascular Surgery by independent investigators in Germany.
New ultrasound method creates better picture of cardiovascular health
Six years ago, a handful of researchers at Lund University in Sweden started taking an interest in how to make it easier to recognise unstable plaques that in worst case scenarios rupture and cause heart attacks or strokes.
First patient enrolled in Xenios i-cor cardiac assist system trial
The first patient has been enrolled in the SynCor clinical trial of Xenios' i-cor synchronised cardiac assist system for treating cardiogenic shock. The SynCor trial is a study of the safety and performance of the i-cor System in 45 consecutively enrolled patients.
US FDA grants Humanitarian Use Device status for Xeltis pulmonary valve
The US Food and Drug Administration has granted Humanitarian Use Device designation for Xeltis' bioabsorbable cardiac pulmonary valve, for the correction or reconstruction of right ventricular outflow tract.
Direct Flow Medical launches next generation TAVI delivery system
Direct Flow Medical has launched the DirecTrack delivery system in Europe. DirecTrack is a next generation delivery system for the Direct Flow Medical transcatheter aortic valve system.
Boston Scientific to present data at American College of Cardiology Scientific Session
Boston Scientific is to present data spanning its interventional cardiology, rhythm management and structural heart portfolios at the American College of Cardiology’s 65th Annual Scientific Session which will be held in Chicago, USA, April 2-4.
Heart attack patients becoming younger and more obese
Despite increased understanding of heart disease risk factors and the need for preventive lifestyle changes, patients suffering the most severe type of heart attack have become younger, more obese and more likely to have preventable risk factors.
Signs of stress in the brain may signal future heart trouble
Signs of stress in the brain have been associated with inflammation in the arteries. Individuals with these signs may be at higher risk for cardiovascular events, including heart attack, stroke and death, according to a study scheduled for presentation at the American College of Cardiology's 65th Annual Scientific Session.
The Medicines Company to present antithrombotic agent data at 2016 ACC meeting
Investigators will present new analyses from high-risk percutaneous coronary intervention (PCI) patient subgroups from the CHAMPION PHOENIX study with The Medicine Company's antithrombotic agent, Kengreal (cangrelor) for injection.
American College of Physicians recommends prescription drug pricing changes
The American College of Physicians (ACP) has released a new policy paper calling for changes that could slow the rising cost of prescription drugs in the USA. The paper was published in Annals of Internal Medicine.
Implantation height affects rate of pacemaker implantation with Sapien 3
Fernando De-Torres-Alba and others report in JACC: Cardiovascular Interventions that a significantly higher rate of pacemaker implantation with Sapien 3 compared with Sapien XT relates to the implantation height of the newer device.
Enrolment complete for MiStent optical coherence tomography study
The MiStent Sirolimus eluting absorbable polymer coronary stent system (MiStent SES) optical coherence tomography (OCT) study has completed enrolment, according to a press release from Micell Technologies.
Stentys Xposition S self-apposing stent receives CE mark for unprotected left main disease
The Xposition S (Stentys), a sirolimus-eluting self-apposing stent, has received CE marking for the treatment of unprotected left main coronary artery disease.
PCI does not reduce readmission rates for patients with syncope and obstructive coronary artery disease
Lindsay Anderson and others report that, compared with medical management, PCI is not associated with a significant reduction hospital readmission for syncope in older patients with syncope and obstructive coronary artery disease.
Sapien 3 now approved for use in Japan
Edwards Lifesciences has announced that the Japanese Ministry of Health, Labor and Welfare (MHLW) has approved its Sapien 3 transcatheter heart valve for the management of severe, symptomatic aortic stenosis.
OptoWire II FFR guidewire receives FDA 510(k) clearance
Opsens has received 510(k) clearance from the FDA for OptoWire II-an optical guidewire developed to measure fractional flow reserve (FFR). The company has already received FDA clearance to sell the OptoWire I, the first generation of its optical guidewire.
First patient receives CorMatrix’s ECM tricuspid valve
According to CorMatrix, a patient has become the first person to receive the company's tissue-engineered regenerative Tricuspid Valve. The operation was performed by Marc Gerdisch, who was implanting the valve as part of CorMatrix's investigational device exemption (IDE)-feasibility study of the device.
Adults with congenital heart disease may be at increased risk of post-traumatic stress
A single-centre study from The Children's Hospital of Philadelphia (CHOP) indicates that as many as one in five adult patients with congenital heart disease have symptoms of post-traumatic stress disorder (PTSD).
The SCOUT study: Transcatheter tricuspid repair
Rebecca Hahn is the principal investigator of the SCOUT trial, which is evaluating the use of Mitralign's transcatheter tricuspid repair system for the management of tricuspid regurgitation. She reviews the potential role of the Mitralign system in treating these patients.
Abbott Vascular recalls MitraClip clip delivery system
Abbott Vascular has recalled the MitraClip clip delivery system, following reports of issues with the delivery system deployment process.
Stenting of narrow pulmonary artery benefits patients with congenital heart disease
The use of a stent to repair pulmonary artery stenosis in children and adults with congenital heart disease was successful in the majority of patients according to new research.
Study suggests no advantages with surgical approach for transfemoral TAVI
James M McCabe and others report that surgical access for transfemoral transcatheter aortic valve implantation (TAVI) is not associated with a significant reduction in major vascular complications compared with percutaneous access.
Magnesium Elektron and Biotronik develop bioresorbable magnesium coronary scaffold
Magnesium Elektron and Biotronik have partnered to develop the SynerMag bioresorbable magnesium alloys for applications in cardiovascular medicine. The joint research and development program began in 2006, with the aim of developing a bioresorbable magnesium coronary scaffold.
Successful PCI of chronic total occlusions does not improve long-term survival
Pil Hyung Lee and others report that successful PCI of a native chronic total occlusion lesion is not associated with improved long-term survival compared with a failed procedure.
Women less likely to receive basic life support for cardiac arrest from public
Women are less likely to receive basic life support for cardiac arrest from members of the public then men according to a study, the European Society of Cardiology announced on the 2016 International Women's Day.
Corvia Medical receives FDA investigational device exemption approval for REDUCE LAP-HF study
Corvia Medical has received investigational device exemption (IDE) approval from the US Food and Drug Administration (FDA) for a multicentre clinical study of the company's InterAtrial shunt device (IASD) for the treatment of heart failure.
Palmaz Scientific seeking prospective buyers following bankruptcy announcement
Palmaz Scientific has filed for chapter 11 bankruptcy protection in San Antonio. According to a company release, this action has been taken to provide adequate time to identify and evaluate prospective buyers for its metallurgical medical device technology.
First patients enrolled in CoreVavle Evolut R real-world study
The first patients have been enrolled in the FORWARD clinical study, which will evaluate performance outcomes associated with the use of the Medtronic CoreValve Evolut in everyday clinical practice, is global, multi-centre, single-arm and prospective. It aims to enrol up to 1,000 patients.
Alexandra Lansky
Alexandra Lansky (Director, Heart and Vascular Clinical Research Program, Yale University School of Medicine, New Haven, USA) talks to Cardiovascular News about what she feels have been the most important clinical breakthroughs in interventional cardiology and what she thinks the next big innovation will be.
Edwards Sapien XT valve granted expanded FDA approval for pulmonic procedures
Adult and paediatric patients presenting with either a narrowed pulmonary valve or moderate or greater pulmonary regurgitation caused by congenital heart disease are now approved for treatment by the Sapien XT valve.
Abbott issues voluntary safety notice on MitraClip delivery system deployment process
The company has received nine Medical Device Reports of malfunction involving MitraClip delivery systems, in which the user was unable to separate the implantable clip from the delivery system.
Long-term results “will prove in favour” of specific device for closing paravalvular leak
A new study has found no significant difference in outcomes between a specifically-designed transcatheter paravalvular leak closure device and other closure devices. Authors claim that the benefit of the specific device will be seen in the long term.
First J-Valve TAVI system implanted outside of China
The J-Valve system (JC Medical) is a next-generation transcatheter aortic valve implantation system, which has been previously used in more than 100 patients in China.
Carillon mitral contour system associated with significant improvement of functional mitral regurgitation
A single-centre study published in the Journal of Invasive Cardiology noted that treatment with Cardiac Dimensions' Carillon mitral contour system resulted in significant improvement of functional mitral regurgitation.
Corindus appoints Mark J Toland as president and chief executive officer
Corindus Vascular Robotics has appointed announced Mark Toland as chief executive officer and member of the Board of Directors. He replaced David Handler, who has announced his resignation. Toland brings over 20 years of experience in the cardiovascular medical device industry.
REVA reaches target patient enrolment in FANTOM II trial
FANTOM II is a clinical study of the safety and performance of the Fantom sirolimus-eluting bioresorbable coronary scaffold. A total of 227 patients have been enrolled at clinical sites in eight countries outside of the USA.
Tirofiban hydrochloride meta-analysis presented at CRT 2016
A meta-analysis of tirofiban hydrochloride (Aggrastat, Medicure) has been presented by primary investigator and lead author, Michael J Lipinski of the MedStar Heart and Vascular Institute, Washington, DC, presented the data at the 2016 Cardiovascular Research Technologies (CRT) conference.
Mitralign percutaneous annuloplasty system granted CE mark for functional mitral regurgitation treatment
The Mitralign percutaneous annuloplasty system (MPAS) has received CE mark approval from the British Standards Institution for the treatment of functional mitral regurgitation (FMR). The product can now be marketed within the European Union.
ReCor Medical receives IDE approval for RADIANCE-HTN hypertension clinical trial
ReCor Medical has been granted US Food and Drug Administration investigational device exemption approval for the RADIANCE-HTN clinical trial. This trial will measure the effect of ReCor's Paradise renal denervation system on blood pressure, in patients with hypertension.
Expanded CE mark approval for Z-Medica QuikClot
Z-Medica has been granted an expanded CE mark for the QuickClot products within the European Union (EU), bringing its indications for use in line with those in the USA.
TherOx adds Keith Grossman to board of directors
Grossman has over 30 years of experience in the medical device field, most recently, and for the second time, as the president, chief executive officer and director of Thoratec Corporation, up to its 2015 sale to St Jude Medical.
Cardiovascular Systems report 30-day results of COAST trial
Thirty-day results of Cardiovascular System's Coronary Orbital Atherectomy System Study (COAST) have been reported at a late-breaking presentation at the 2016 Cardiovascular Research Technologies Conference in Washington, USA.
Second USA minimally invasive Trialign procedure performed in Chicago
Interventional cardiologists at Northwestern Memorial Hospital, Chicago, USA have become the first in the mid-west of the USA, and the second nationally, to perform a minimally invasive tricuspid valve procedure using the Mitralign Trialign system.
First UK Revivent-TC transcatheter ventricular enhancement procedure performed
The first clinical use of BioVentrix's closed-chest Revivent-TC transcatherter ventricular enhancement system in the UK has taken place at Freeman Hospital, Newcastle-upon-Tyne. The less invasive ventricular enhancement (LIVE) procedure is used in the treatment of ischaemic cardiomyopathy.
Complete PCI in STEMI: An interventional paradigm shift
In a new focused update, the ACC/AHA/SCAI now recommend that complete PCI is acceptable in some patients with STEMI with multivessel disease. Eliano P Navarese reviews the evidence base for complete PCI compared with culprit-artery only PCI.
Medtronic can now investigate CoreValve Evolut R in low-risk patients
Medtronic has announced that the FDA has approved an expanded indication trial for the CoreValve Evolut R system, which enables the TAVI device to be investigated in patients with aortic stenosis who are at a low surgical mortality risk as determined by a heart team.
First patient enrolled in study of supersaturated oxygen system for acute myocardial infarction
The investigational device exemption confirmatory study will investigate the ability of SSO2 therapy to reduce infarct size after AMI, and is being conducted to support a premarket approval submission to the US Food and Drug Administration.
Effects of the Paris attacks on cardiovascular hospitalisation in Toulouse
Observing a sharp increase in admissions in their cardiovascular unit directly following the 2015 Paris attacks, Atul Pathak and his team hypothesised that the sudden increase of activity in their department might be stress-induced.
First two patients treated in study of Peregrine system for renal denervation
The first patients were treated at an American Heart of Poland hospital in Poland, using the Peregrine system infusion catheter (Ablative Solutions) with a neurolytic agent to treat sympathetic nerves in the outer layer of the renal arteries.
Direct Medical Flow announce transcatheter mitral valve development program
The mitral valve is designed to feature a low atrial profile, low ventricular projection and conformable sealing and fixation rings for the complex mitral annulus. This announcement took the form of a preclinical case presentation of the Transcatheter mitral valve.
Heart attack patients with cardiogenic shock fair well from 60 days post-discharge
Heart attack patients who experience cardiogenic shock have a higher risk of death or rehospitalisation than non-shock patients in the first 60 days post-discharge. By the end of the first year, however, the gap between the two groups narrows, according to a new study in the Journal of the American College of Cardiology.
Makkah’s cardiac hospital describes how it copes with yearly pilgrimage
There is a huge influx of patients every year to Makkah (Mecca), Saudi Arabia, during the Hajj. Makkah's cardiac hospital describes how it copes with this, and gives details of its echocardiography service in an abstract presented at the 27th Annual Conference of the Saudi Heart Association.
Acute vessel wall injury is common after transradial catheterisation but is not linked to radial artery occlusion
Acute vessel wall injury is common after transradial catheterisation but it is not associated with an increased risk of radial artery occlusion or loss of radial artery pulsation, according to a report published in Circulation: Cardiovascular Interventions.
More than 200 patients have been enrolled in Reva Medical’s study of Fantom device
Reva Medical has announced that more than 200 patients have been enrolled in its clinical study of its sirolimus-eluting bioresorbable coronary scaffold (Fantom).
NeoChord receives German NUB status 1 for DS1000 mitral valve repair system
The NUB (Neue Untersuchungs- und Behandlungsmethoden) process enables the introduction of new medical products prior to formal reimbursement eligibility and introduces the cost of a new procedure into the German reimbursement system.
European Society of Cardiology announces winners of innovative cardiovascular research grant programme
The 'Grants for Medical Research Innovation' are awarded to research projects that will address areas of unmet medical need in thromboembolic disease.
CorMatrix Cardiovascular receives 510(k) clearance for the Tyke implantable device for neonate and infant cardiac tissue repair
CorMatrix Cardiovascular has received 510(k) clearance from the US Food and Drug Administration for its CorMatrix Tyke, a biomaterial technology derived from the company's extracellular matrix biomaterial technology platform.
Valtech Cardioband reconstruction system for mitral valve repair receives reimbursement in Germany
Valtech has received German Neue Untersuchungs und Behandlungsmethoden (NUB) Status 1 approval for the Cardioband mitral reconstruction system, its flagship device for addressing mitral regurgitation in heart failure patients.
FDA Advisory Committee to review AngelMed Guardian system for pre-market approval
The Circulatory System Devices Panel of the US Food and Drug Administration will review Angel Medical Systems' premarket approval application for the AngelMed Guardian system at a meeting on the 16 March 2016.
Admedus appoints Wayne Peterson as new chairman of the Board
Wayne Paterson has been appointed as non-executive chairman of the Admedus Board of Directors, with immediate effect. This follows the resignation of Chris Catlow who held the position for five years.
First patient enrolled in trial of the COBRA PzF nanocoated coronary stent system
The first patient has been enrolled in CeloNova BioSciences' COBRA REDUCE trial, which will study the Cobra PzFTM nanocoated coronary stent system in patients at a high risk of bleeding. The trial recently received conditional US Food and Drug Administration approval.
St Jude Medical launches Optis mobile system in Japan and Europe
The diagnostic system is designed to couple optical coherence tomography (OCT) and angiography co-registration with fractional flow reserve (FFR) technology into one portable system for hospitals with multiple catheterisation labs.
US Medicare to cover Boston Scientific Watchman left atrial appendage closure device
The US Centers for Medicare and Medicaid Services (CMS) are to cover percutaneous left atrial appendage closure (LAAC) therapy under specific criteria, as outlined in the agency's final National Coverage Determination (NCD).
Corindus appoints Campbell Rogers to its Board of Directors
Rogers has served as chief medical officer of HeartFlow since 2012. He has also conducted extensive clinical research including serving as principal investigator for numerous interventional cardiology device, diagnostic and pharmacology trials.
Clive Meanwell of The Medicines Company receives innovation and leadership award
Clive Meanwell, the founder and chief executive of The Medicines Company, has received the 2016 Sol J Barer Award for Vision, Innovation and Leadership.
Building the ideal heart team for transcatheter mitral valve interventions
In this commentary, Vinayak Bapat outlines transcatheter mitral valve interventions and reviews which specialists should comprise the optimal heart team for such procedures.
Alexander J Denner appointed to The Medicines Company Board of Directors
Denner will serve as a class III director, with a term expiring at the company's 2018 Annual Meeting of Stockholders. He will also serve on the Company's Nominating and Corporate Governance Committee.
CorMatrix Cardiovascular prosthetic heart valve platform receives 27th patent
CorMatrix Cardiovascular's prosthetic heart valve platform has been issued US Patent No. 9,226,821, bringing the total number of issued patents relating to this platform to 27. Thirty patents are pending in the US and internationally.
Admedus CardioCel achieves first sales in Middle East and North Africa region
The initial sales of CardioCel have come through an early access program in Qatar. Admedus anticipates additional sales in other MENA countries in the near future.
New sizes of the Medtronic launches new Resolute Onyx drug-eluting stent sizes
The new sizes-4.5mm and 5mm-have recieved CE mark, along with several new product indications including treatment of left main vessels and small vessels.
OrbusNeich highlights new generation coronary balloons at AsiaPCR/SingLIVE 2016
The Case-in-Point session, 'Your complex PCI: How can new technologies enhance procedural success?', reviewed solutions for calcified lesions with complex anatomy, where the dual wire Scoreflex balloon can be used as an alternative to rotablation.
Admedus signs distribution agreement with Coroneo
Admedus has entered into an exclusive distribution agreement with Canadian company Coroneo to sell its Aortic Annuloplasty Ring and a range of specialised surgical instruments for heart valve surgery in Germany and the UK.
Boston Scientific and Accenture develop data-driven digital health platform to improve efficiency and patient outcomes
The platform, called Advantics Care Pathway Transformation, is designed to help improve patient outcomes and reduce costs to treat patients with chronic cardiovascular diseases.
New AHA statement says symptoms of myocardial infarction may differ in women
In a new scientific statement, the American Heart Association advises that the causes, symptoms, and outcomes of myocardial infarction may be different in women compared with men. It adds that these differences are further pronounced in Black and Hispanic women
Non-transfemoral access is strongly associated with post-TAVI delirium
Masieh Abawi and others report in JACC Cardiovascular Interventions that about 13% of patients develop postoperative delirium after undergoing transcatheter aortic valve implantation (TAVI).
Roxwood Medical launches Micro14es extra support catheter
Micro14es is an extension of the Micro14 catheter, intended for complex peripheral and coronary disease. It is designed to provide enhanced guidewire support through challenging and tortuous anatomy.
RenalGuard Solutions announces new European clinical trial to evaluate long-term impact of RenalGuard therapy in patients at high risk for CI-AKI
RenalGuard Solutions has announced the STRENGTH trial, designed to evaluate the long-term clinical and economic impact of RenalGuard therapy in patients at high risk for contrast-induced acute kidney injury (CK-AKI), which will begin later this year.
JenaValve Technology appoints Pieter Van den Steen as chief commercial officer, general manager
Prior to his current appointment, Van den Steen served as the commercial leader of Boston Scientific's Peripheral Interventions division in Europe, after being the general manager for one of their European regions.
The patient mind: Before, during & after heart surgery
In this Heart Valve Surgery Resources video, Kim Feingold and Duc Thinh Pham (both Northwestern Medicine, St Albans City, USA) discuss cardiac behavioural medicine for patients undergoing heart valve surgery.
Training is the key to wider adoption of the transradial approach for PCI in the USA
This commentary explores why the USA, despite the data supporting the transradial approach, has been more reluctant than other countries to adopt the approach.
Reva hires Richard Kimes as senior vice president of Operations
Richard Kimes has joined Reva Medical as the company's senior vice president of operations. He is now responsible for leading manufacturing-related activities in support of the launch of sirolimus-eluting bioresorbable coronary scaffold, Fantom, in international markets.
Svelte Medical Systems announces European launch of Slender IDS
The Slender sirolimus-eluting coronary stent-on-a-wire integrated delivery system is to be launched commercially to select European accounts specialising in transradial intervention.
Valentin Fuster awarded Spain’s highest civilian award for services to healthcare
Valentin Fuster, editor-in-chief of the Journal of the American College of Cardiology has been awarded the Gran Cruz de la Orden Sanidad (Grand Cross of the Civil Order of Health) by the Spanish government.
One-hour diagnosis of heart attack possible with Roche troponin T test
Data from the TRAPID-AMI clinical study have confirmed the troponin T high-sensitivity test from Roche as an approach which can offer a more rapid diagnosis of heart attack in patients with acute chest pain.
Conavi Medical receives FDA 510(k) clearance for Foresight intracardiac echocardiography system
The product has been cleared for intracardiac and intraluminal ultrasound visualisation of cardiac and great vessel anatomy, as well as visualisation of other devices in the heart and great vessels of patients.
Edwards can expand Sapien 3 study indications following FDA approval
The FDA has given Edwards Lifesciences approval for an expanded indication study of its Sapien 3 valve. The IDE study will enrol elderly patients with severe, symptomatic aortic stenosis who are at low risk for surgery.
Expert consensus statement for treatment of cardio-oncology patients released by the Society for Cardiovascular Angiography and Interventions
The statement is intended to provide cardiologists, oncologists and internal medicine physicians with guidance for treating patients facing both cardiovascular disease and cancer.
Japanese Ministry of Health, Labour and Welfare approve Occlutech atrial septal defect closure device
This product is indicated for the minimally invasive closure of atrial septal defects (ASD). The approval has been made possible by a partnership between Occlutech and Japan Lifeline.
SurModics acquires NorMedix, a company focused on minimally invasive catheter technologies
This acquisition is intended to bolster SurModic's vascular device expertise and research and development capabilities.
Retrograde coronary revascularisation in Europe to treat chronic total coronary occlusions
Alfredo R Galassi reports on the use of PCI with the retrograde approach for the management of CTOs in Europe and how the Euro CTO Club and Live Summit are spreading the knowledge of new complex PCI techniques for CTOs.
The Medicines Company advances clinical development of investigational PCSK9 synthesis inhibitor for atherosclerotic cardiovascular disease
The Medicines Company has initiated study sites, and begun enrolling patients, in the ORION-1 phase 2 study. ORION-1 will compare the effects of differenct ALN-PCSsc doses.
HeartWare appoints Stephen Oesterle to board of directors
HeartWare International has appointed Stephen Oesterle to its board of directors. Oesterle's career in cardiology and medical devices has spanned over 30 years, including senior leadership roles at Medtronic.
Vivasure granted CE mark for world’s first fully bioabsorbable percutaneous closure device for large-bore transcatheter
Vivasure Medical has been granted CE mark approval for its fully bioabsorbable percutaneous vascular closure device for large-bore femoral arteriotomies.
Clinical results of Chocolate Heart drug-coated coronary balloon announced by QT Vascular
QT Vascular and its subsidiaries have announced the release of the initial results from the first-in-human (FIH) study of its unique drug-coated balloon, Chocolate Heart.
New coronary congenital disease classification suggested to aid identification of secondary defects
A new classification of coronary congenital diseases has been suggested to help surgeons identify secondary defects in the operating theatre. The scheme is outlined in a novel European Society of Cardiology (ESC) position paper published today in Cardiovascular Research.
Orsiro may provide benefit over Xience for STEMI patients
A pre-specified substudy of BIOSCIENCE, published in EuroIntervention, has found that Orsiro is associated with a significantly lower rate of target lesion failure than is Xience in STEMI patients at one year.
Hyperglycaemia could lead to heart attack complications
The mechanism by which blood glucose levels can affect the contraction of blood vessels has been demonstrated for the first time by a team from the University of Leicester, UK.
Longer treatment times found for heart attack patients with history of bypass graft surgery
Heart attack patients who have previously had coronary artery bypass graft surgery may be less likely than other heart attack patients-including those with prior angioplasty-to be treated within the recommended "door-to-balloon" time.
Seeking treatment earlier may improve heart attack outcomes
Patient delays have been associated with an increase in damage to the heart, according to a study published online in JACC: Cardiovascular Interventions.
Risk of cardiovascular events and death minimised by greater reduction of blood pressure than current guidelines recommended
Researchers have recommended that blood pressure-lowering drugs should be offered to all people at a high risk of heart attack of stroke, regardless of their blood pressure at the start of treatment.
MicroPort EP completes first clinical case observation on its cardiovascular catheter sheath
MicroPort EP completes first clinical case observation on its cardiovascular catheter sheath
Coronary heart disease patients with no teeth have nearly double the risk of death
A study from Uppsala, Sweden has found a linear association between levels of tooth loss and increasing death rates across 15,000 patients from 39 different countries.
HeartWare International receives director nominations notice from Engaged Capital
The company has also responded to comments from Engaged Capital, a new stockholder, regarding its proposed acquisition of Valtech Cardio.
AdvaMed, MITA & MDMA applaud congress for passage of the medical device tax suspension
The Advanced Medical Technology Association (AdvaMed), the Medical Imaging & Technology Alliance (MITA) and the Medical Device Manufacturers Association (MDMA) have applauded the US Congress for their passage of a two year suspension of the medical device excise tax, in year-end legislation.
Edward Heart Hospital becomes first in Illinois, USA to use CorPath system for robotic-assisted vascular procedures
The CorPath system is designed to allow interventional cardiologists to perform procedures away from the patient bed side and the radiation source.
OrbusNeich’s new generation coronary balloons showcased at GulfPCR-GIM 2015
OrbusNeich presented the Sapphire II Pro and Scoreflex products at a case-in-point session at the course in Dubai, UAE.
GulfPCR explores transformative learning
Jean Marco, PCR honorary chairman, discussed "transformative learning" during a keynote lecture at the 2015 GulfPCR meeting.
The role of psychological support after a myocardial infarction
This commentary reviews the benefits of providing psychological support for patients who experience emotional distress after having a myocardial infarction.
Radial access used less than femoral approach for emergency angioplasty
This is in spite of the fact that using the radial artery as the access point for angioplasty is associated with less bleeding than use of the femoral artery.
Xeltis extends series B financing to €30 million
This €3 million extension is intended to hasten Xeltis' pipeline development on a number of cardiovascular indications.
CE mark for Svelte Medical’s sirolimus-eluting coronary stent-on-a-wire integrated delivery system
Svelte Medical Systems has received the CE mark for its Slender sirolimus-eluting coronary stent-on-a-wire integrated delivery system for the treatment of coronary artery disease.
Gregory D Casciaro becomes president and chief executive officer of Cardiac Dimensions
Cardiac Dimensions has announced the appointment of Gregory D Casciaro as president and chief executive officer, effective immediately. He will also become a member of Cardiac Dimensions' board of directors.
Mind the gender gap: Addressing inequalities in interventional cardiology
With only 4.5% of US interventional cardiologists being female, Cardiovascular News reviews the steps being taken to ensure that being female is not a barrier to being an interventional cardiologist.
Optimism associated with reduced cardiac readmission after acute coronary syndrome
Optimism after acute coronary syndrome has been found to be an independent predictor of both increased physical activity and reduced cardiac readmission. However, gratitude does not appear to affect these outcomes.
Roxwood Medical announces full US launch of its MicroCross catheter
Roxwood Medical has said now that more than 500 patients have been successfully treated with its MicroCross catheter (as part of an initial limited release), it is now going to fully launch the catheter in the USA
Edward Lifesciences announces further investments into mitral valve therapies
Edwards Lifesciences announced a structured investment in Harpoon Medical, which is pioneering a beating-heart, transcatheter therapy for minimally invasive surgical repair of a degenerative mitral valve.
i-cor synchronised cardiac assist preserves left ventricular function compared with continuous-flow ECLS in cardiogenic shock
A new study indicates that the i-cor synchronised cardiac assist system protects left ventricular function compared with continuous-flow extracorporeal life support in cardiogenic shock.
New study will evaluate Heart Test Laboratories’ MyoVista heart screening device
Heart Test Laboratories has announced the start of a clinical study of its MyoVista device by Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center (LA BioMed).
US cardiovascular societies publish joint expert consensus on left atrial appendage occlusion requirements
SCAI, ACC, and HRS have published a joint document providing recommendations for the establishment and maintenance of left atrial appendage occlusion programmes performing LAA closure.
Enrolment completed in randomised trial of MiStent and Xience
Stentys has announced that patient enrolment in its DESSOLVE III study, which is comparing the MiStent sirolimus-eluting stent with the Xience everolimus-eluting stent, has been completed.
Antonio Colombo joins Scientific Advisory Council of Miracor Medical Systems
Antonio Colombo (Cardiac Catheterization Laboratory, EMO GVM Centro Cuore Columbus; San Raffaele Hospital, Milan, Italy) has been appointed as a member of the Scientific Advisory Council of Miracor Medical Systems.
Survey suggests that cardiology is one of the rudest specialties
Victoria Bradley and others report that cardiology is among the departments/specialists seen as most likely to engage in rude, dismissive and aggressive behaviour towards colleagues.
Promising results for novel wave membrane blood pump
CorWave has reported promising early results for its left ventricle assist device (LVAD). The results were presented at 10th European Mechanical Circulatory Support meeting (EUMS; 2-5 December, Paris, France).
BioVentrix Announces first interventional heart failure procedure for left ventricle volume reduction in a pre-clinical model
The first implantation of BioVentrix' micro-anchor technology entirely within the left ventricle using a catheter-based endovascular approach has been successfully completed.
Medinol US Commercialises Novel Coronary Stent
Medinol continued its introduction of its novel stent solutions during the 2015 Transcatheter Cardiovascular Therapeutics (TCT) meeting in San Francisco.
Happiness has no direct effect on mortality
A study of a million UK women has shown that the common belief that happiness and stress directly cause ill health has been fuelled by the confusion of cause and effect in previous studies.
Interventional cardiologists may have “a false sense of security” about radiation safety
Michael Seymour, director of Advocacy Programs for ORSIF, believes that there needs to be greater awareness of the dangers of radiation exposure during interventional procedures as some interventional cardiologists may not appreciate the true risks that they face in the cath lab.
Aortic stenosis: Future is Now!
Olcay Aksoy discusses how transcatheter aortic valve implantation (TAVI) has revolutionised the treatment of patients with aortic stenosis.
Arterys partners with GE Healthcare to launch new cardiac imaging platform
The Arterys system is an intelligence platform designed to enhance standard medical imaging by enabling clinical visualisation and accurate quantification of blood flow inside the body.
Previous guidelines were “overconfident” about benefits of P2Y12 inhibitor pre-treatment
New European Society of Cardiology (ESC) guidelines for the management of patients who present with non-ST-segment elevation acute coronary syndrome were revealed at the 2015 ESC Congress (29 August-2 September, London, UK) and simultaneously published in European Heart Journal. The chairperson of the guidelines Marco Roffi (Interventional Cardiology Unit, University Hospital, Geneva, Switzerland) outlines the main differences between these guidelines and previous guidelines.
New European code says no to direct sponsorship of physicians to attend congresses but says yes to sponsorship via healthcare organisations
The European Diagnostics Manufacturers Association and the European Medical Technology Industry have approved a new joint code of conduct that stipulates that, after 31 December 2017, industry should no longer provide direct "financial or in kind support" to individual healthcare professionals to cover the costs of them attending third-party medical conferences.
First patient enrolled in Mitralign’s US percutaneous tricuspid repair study
The first US patient has been enrolled in Mitralign's SCOUT study, which is evaluating the company's percutaneous tricuspid valve annuloplasty system (Trialign).
MedStar Heart and Vascular Institute at MedStar Washington Hospital Center to start TAVI trial in low-risk patients
The FDA has given IDE approval to MedStar Heart and Vascular Institute at MedStar Washington Hospital Center (Washington, DC, USA) to start a new study of TAVI in low-risk patients.
Heartbeat International foundation and Biotronik open new centre in the Dominican Republic
The Heartbeat International foundation and Biotronik have opened a new heart centre in the Dominican capital of Santo Domingo. At the centre, patients will be able to received donated pacemakers and defibrillators.
Personally tailored diabetes care reduces mortality in women but not men
This structured approach could conflict with men's tendency to trust self-directed learning instead of self-management, say study authors.
New delivery sheath may enable more patients to undergo transfemoral TAVI
Walid K Abu Shaleh and others report in Catheterization and Cardiovascular Interventions that a new balloon-expandable, re-collapsible (BERC) sheath may "considerably expand" the population suitable for TAVI via the transfemoral approach.
The rewards of revascularising a CTO
J Aaron Grantham describes the effect of using PCI to treat a patient with a CTO of the left anterior descending coronary artery.
Helping Little Hearts to mend
Nurun Nahar Fatema talks about how Muntada Aid's Little Hearts campaign has helped children in Bangladesh with congenital heart problems.
Medical titles are “hierarchical” and “hardly appropriate” for person-centred care
In a comment piece in the British Medical Journal, Ashley Graham Kennedy argues that title "doctor" is hierarchical because it suggests that a patient is lesser than and, should defer to, a physician.
First patient undergoes mitral replacement with NaviGate Cardiac Structures’ mitral valve stent
NaviGate Cardiac Structures has revealed that the first-in-human implant of its catheter-guided, mitral-valve stent into a beating heart was performed successfully in a 53-year-old male patient presenting with severe mitral regurgitation.
Sedentary patients with heart disease have worse health even if they exercise
Patients with heart disease who sit a lot have significantly worse body mass index and cardiorespiratory fitness, even if they exercise.
Vascular Solutions’ R350 guidewire receives 510(k) clearance
The new R350 uses a nitinol core, designed to provide superior flexibility and kink-resistance in extreme tortuosity, and a hydrophilic coating on the distal 200cm.
i-cor synchronised cardiac assist system recieves CE mark
The product by Xenios AG can now be sold in Europe as the world's first heartbeat-synchronised cardiac assist for cardiogenic shock and high-risk interventions.
Biosense Webster acquires Coherex Medical
Biosense Webster has announced that it has acquired Coherex Medical, developers of the...
FDA issues safety communication on lubricious coating separation from intravascular medical devices
The communication includes guidelines for healthcare providers treating patients during or after intravascular procedures.
Occurrence of surgical ‘never events’ unrelated to hospital performance
A study has found that the size of individual hospitals is the only factor influencing their rate of 'never events'.
Next-generation Watchman approved for use in Europe
The next-generation Watchman left atrial appendage closure device (Watchman FLX) has received CE mark approval and the first implants of the device have taken place in Europe, commencing limited market release.
Anticoagulation with heparin “should remain standard of care” during TAVI procedures
George Dangas (New York, USA) and others that heparin should continue to be the first-line anticoagulant for TAVI procedures because it is not associated with a significantly increased risk of bleeding compared with bivalirudin.
Thrombus aspiration does not improve 12-month outcomes according to TATORT-NSTEMI trial
TATORT-NSTEMI is the first randomised trial investigating the impact of thrombectomy before percutaneous coronary intervention.
American Heart Association and Google Life Sciences to collaborate on US$50 million research project
The AHA and GLS will invest US$25 million each over roughly five years to support a research collaboration targeting coronary heart disease and its consequences.
Moderate coffee consumption may be good for you
According to a study published in Circulation, people who regularly drink moderate amounts of coffee daily (less than five cups per day) have a lower risk of deaths from cardiovascular disease.
Cardiovascular Systems CEO to take medical leave
David L Martin, chief executive officer of Cardiovascular Systems is due to take approximately three months to focus on treatment for cancer.
Enrolment begins in the ACIST-FFR multi-centre study
The purpose of the ACIST-FFR study is to advance clinical understandings of FFR technologies. It will investigate measurement accuracy, incidence of drift and device success.
COURAGE: No long-term benefit with PCI in stable disease
Steven P Sedlis and others that 15-year follow-up data from COURAGE indicate that PCI does not significantly improve survival compared with optimal medical therapy in patients with stable coronary artery disease.
SentreHeart to present data on Lariat treatment for atrial fibrillation at LAA Conference
The research is intended to demonstrate that a Lariat procedure for LAA closure, plus a subsequent PVI catheter ablation, will lead to a reduced incidence of recurrent AFib compared to PVI alone.
New risk score could aid decision making about extending dual antiplatelet therapy beyond 12 months
Robert W Yeh (Boston, USA) told delegates at the 2015 AHA scientific sessions that a new risk score could be used to predict which patients may benefit from extending dual antiplatelet therapy beyond 12 months.
Fewer “inappropriate” PCI procedures are being performed in the USA
A new study indicates that following the introduction of appropriate use criteria for coronary revascularisation, there has been a significant reduction in number of inappropriate PCI procedures that are performed in the USA.
Study shows extended-release aspirin capsules can deliver sustained antiplatelet effects for 24 hours
The New Haven Pharmaceuticals study analysed the effects of the company's "Durlaza" extended-release aspirin.
New transcatheter heart valve manufacturing facility certified for JenaValve
The wholly-owned facility is equipped to develop, test and deliver existing and improving valve technologies
Discussion on the DAPT score
In this ACC/MedPage Today video, Peter Block and Robert Yeh discuss the DAPT score, which Yeh presented at the 2015 AHA scientific sessions.
An interventional cardiologist’s view on left atrial appendage closure
Martin W Bergmann is an interventional cardiologist working in Germany, who performs percutaneous LAA closure procedures. He explores why there is still a need for such procedures even in the era of novel oral anticoagulants.
Protein reprogramming method might yield rich source of heart cells for cardiac repair
A study has reported an efficient protein-based method for converting fibroblasts into cardiac progenitor cells (CPCs) directly.
WomenHeart appoints three new members to its board of directors
WomenHeart: The National Coalition for Women with Heart Disease has appointed three women heart disease survivors.
PEGASUS-TIMI 54 subanalysis provides additional insights into long-term ticagrelor use
The PEGASUS-TIMI 54 subanalysis evaluated reasons and rates for discontinuation of ticagrelor in patients who had experienced a myocardial infarction one to three years prior to study randomisation, and the efficacy in those patients who stayed on therapy.
3D system may more accurately identify best donor hearts for a paediatric transplant patients
Researchers have created a novel library of healthy children's 3D reconstructed hearts using MRI and CT images.
Women cardiologists specialise in different areas, and are paid less, than men
Over a lifetime of work, women cardiologists earn more than US$1 million less than their male counterparts.
North Carolina scientist awarded American Heart Association Population Research Prize
The prize was in recognition of his findings of artery wall predictors of cardiovascular disease risk.
New long-term data demonstrates benefit of CardioMEMS HF system over standard therapy
Patients from the study's control group had a 48% reduction in HF hospitalisations after moving from standard HF therapy to the CardioMEMS HF System.
Group therapy can promote cardiovascular health, according to study
Peer group support has been found to improve healthy behaviour in people with cardiovascular risk factors.
Wealthiest patients may have edge over sickest in organ transplants
The ability to register with more than one organ transplant centre seems to increase a patient's likelihood of receiving a transplant.
Xeltis completes second feasibility clinical trial on bioabsorbable cardiovascular technology
The trial has shown positive results in patients a year after surgery for Xeltis' products.
EMA grants CardioCel a broader indication for use in Europe
The product is now approved for use in the repair and construction of heart valves.
World’s largest cardiovascular imaging conference to showcase technological innovations
Innovations in echocardiography, cardiovascular magnetic resonance (CMR), nuclear cardiology and cardiac computed tomography (CT) will be presented and discussed during the meeting.
TherOx granted FDA IDE Approval to study next-generation supersaturated oxygen therapy for AMI
This study-which will recruit 100 patients-is taking place to support a pre-market approval (PMA) application to the FDA.
Third-generation renal denervation system by ReDy receives CE mark approval
Based on a novel multi-ablation technology, ReDy claim that its renal denervation system delivers a pre-determined RF ablation with a single positioning.
MC3 Cardiopulmonary acquires surgical cannula product assets from Terumo Cardiovascular Systems
The company has built a 60,000-square-foot manufacturing facility in Dexter, USA, to bring the Sarns Soft-Flow arterial cannula back to market in 2016.
New data on The Medicines Company’s cardiovascular portfolio to be presented at AHA
The company has announced it will present late breaking clinical trial data on ALN-PCSsc, as well as three abstracts on the antithrombotic cangrelor (Kangreal).
Minimally invasive, leadless endovascular neuromodulation system introduced by Enopace Biomedical
The Harmony catheter-based neurostimulator device system is designed to treat congestive heart failure.
Custom-designed vascular training model now available at events
The ThinkRadial Vascular Training Model provides realistic simulation to several procedurally critical vascular destinations, while allowing the practitioner to "see" the way radial products perform inside the body.
Medeon Biodesign’s XPro large bore vascular closure device achieves successful first-in-man studies
The device's percutaneous approach is designed to be less traumatic for patients and to achieve shorter recovery times.
CardioCel bio-scaffold launched and sold in Malaysia
The launch is one stage of Admedus' ongoing global rollout of Cardiocel, which has now been implanted in over 3,000 patients.
American College of Physicians joins brief urging Supreme Court to uphold considerations of race, ethnicity in medical school admissions process
The petitioners argue that health disparities continue along the lines of race and ethnicity, and that health profession needs to be culturally competent.
Low baseline albumin associated with increased all-cause mortality after TAVI
A new study indicates that low baseline albumin (_4g/dl) in patients undergoing TAVI is associated with a four-fold increase in all-cause mortality at one year and a two-fold increase in all-cause mortality at two years.
Tryton Medical files pre-market approval application with US FDA for Tryton Side Branch Stent
The stent could become the first bifurcation stent available in the USA if approved.
SCAI calls for abstract submissions
Roxana Mehran encourages people to attend the 2016 Society for Cardiac Angiography and Interventions meeting
The risk of stroke in patients undergoing TAVI
While TAVI is the standard of care for inoperable patients with severe symptomatic aortic stenosis and a compelling approved alternative to surgical aortic valve replacement for patients at high operative risk, the early experience with the intervention raised concerns regarding the risk of stroke and other neurological events. Alexandra Lansky and Cody Pietras explore the risk of stroke in patients undergoing TAVI.
PiCSO highlighted at TCT as novel therapy
Miracor Medical Systems have reported that, during this year's TCT meeting, PiCSO has been highlighted in several sessions as offering new hope for cardioprotection during percutaneous coronary intervention.
Opsens performs 1,000th procedure with the OptoWire
Opsens has also begun pre-commercialisation activities for its Fractional Flow Reserve products.
Death after heart surgery overwhelmingly determined by patient risk factors
A study published in Anaesthesia has shown that death after heart surgery is not determined by which professionals or hospitals provide the care.
Direct Flow Medical appoints new co-principal investigator for US SALUS pivotal trial
Direct Flow Medical has appointed D Scott Lim as national co-principal investigator for the SALUS pivotal trial. He joins co-principal investigator Isaac George.
In-hospital mortality higher for young women after STEMI
Young women with ST-elevation myocardial infarction spent more days in hospital, had higher rates of in-hospital mortality and were less likely to receive angioplasties and stenting than men, according to a study published in the Journal of the American College of Cardiology (JACC).
Angioplasty balloon catheter from AV Medical Technologies receives FDA clearance
AV Medical Technologies has announced that it has received U.S. Food and Drug Administration (FDA) clearance for its Chameleon angioplasty balloon catheter.
Biotronik announces European launch of new generation of Galeo coronary guide wires
A press release from Biotronik has announced the European market launch of a new range of Galeo guide coronary workhorse guide wires, inspired by the original Galeo design.
Curbing atherosclerosis and precision medicine should be main strategies to transform the landscape of treatments to prevent heart attacks and strokes
According to research presented at the Canadian Cardiovascular Congress (CCC) by Jean-Claude Tardif, personalised therapy should become a main strategy in the treatment and prevention of heart disease and stroke.
Edwards Lifesciences appoints Martha Marsh to board of directors
Edwards Lifesciences has announced that Martha H Marsh has been appointed to its board of directors. Currently, Marsh serves on the boards of directors of AMN Healthcare Services and Owens & Minor.
New guidance issued for multivessel PCI, thrombectomy in heart attack patients
A focused update has been issued offering new guidelines on the use of multivessel coronary interventions and thrombectomy in patients with heart attacks due to completely blocked arteries, from American College of Cardiology, the American Heart Association, and the Society for Cardiovascular Angiography and Interventions, in collaboration with the American College of Emergency Physicians.
Spiral Flow PV bypass graft launched by Vascular Flow Technologies
The graft was launched at the 31st annual Deutschen Gesellschaft fÌ_r GefÌ_ÌÙmedezin (DGG) in MÌ_nster, Germany. Earlier this month, it was implanted in a live case during the Vascular/Endovascular Masterclass in Hamburg, Germany.
Further evidence needed for clinical consequences of reduced leaflet motion
A new study indicates that reduced leaflet motion occurs with all types of bioprosthetic valves and may be linked to an increased risk of stroke. Therefore, the study investigators state that further studies are needed to better characterise the clinical consequences of reduced leaflet motion.
Antiplatelet therapy with blood thinners reduces mortality for angioplasty patients
Glycoprotein 2c/3a inhibitor (GPI) use in patients following angioplasty procedures has been associated with a reduced risk of all-cause in-hospital mortality, but an increased risk of bleeding.
Corrado Tamburino
Corrado Tamburino (full professor of Cardiology, Ferrarotto & Policlinico Hospitals, University of Catania, Catania, Italy), and a course director for PCR London Valves has done extensive work in developing PCI techniques for left main disease
Use of fusion imaging during TAVI
Mehdi Eskandari and Mark Monaghan explore the use of TOE and fluoroscopy during TAVI, focusing on how fusion imaging may help to improve safety and accuracy during these procedures.
British Heart Foundation announces strategy for over half a billion pounds of research funding
The British Heart Foundation (BHF) has launched a five-year strategy for research, including a commitment to sustain funding for the best researchers across all areas of cardiovascular disease (CD).
One-year outcomes from OrbusNeich’s Combo REMEDEE all-comers registry demonstrate clinical effectiveness in target lesion failure
REMEDEE is a 1000-patient registry designed to evaluate the Combo Dual Therapy Stent for the treatment of coronary lesions in the routine clinical care setting. The outcomes demonstrate clinical effectiveness in one-year target lesion failure.
Neovasc Tiara featured in live case broadcast at TCT 2015 meeting
In the broadcast to the main arena of the conference, a 35mm Tiara transcatheter mitral valve was successfully implanted in a patient with severe mitral regurgitation within thirty minutes, with no procedural complications.
Edwards Sapien 3 valve demonstrates high survival and low stroke rate at one year
Edwards Lifesciences has announced a one-year survival rate of 89.3% for high-risk patients who received the Edwards Sapien 3 transcatheter aortic valve via transfemoral, as well as low rates of paravalvular leak and stroke.
SHIELD I trial results confirm consistent cardiac support offered by St. Jude Medical HeartMate PHP cardiac assist device during high-risk PCI procedures
The data show that the hemodynamics of severely ill patients undergoing complex revascularisation procedures can be maintaimed by use of the HeartMate PHP cardiac assist.
Lesion preparation with scoring balloon may improve performance of drug-coated balloons in managing in-stent restenosis
ISAR-DESIRE 4, which is the first trial to examine the role of lesion preparation before using drug-coated balloons, indicates that the use of a scoring balloon may enhance the efficacy of drug-coated balloons to treat in-stent restenosis.
Edwards Sapien XT valve granted FDA approval for aortic valve-in-valve procedures
Edwards Lifesciences has announced US FDA approval has been granted for aortic valve-in-valve procedures using its Sapien XT transcatheter heart valve. This follows the presentation of high survival and low stroke rates from the one-year outcomes of the PARTNER II Valve-in-Valve Study.
Sentinel cerebral protection system provides definite cognitive benefit for TAVI patients
Results from the MISTRAL-C study indicate that use of the Sentinel cerebral protection system (Claret Medical) during TAVI procedures is associated with definitive cognitive benefit. The data were presented at TCT.
“Excellent” one-year outcomes for Direct Medical TAVI valve
press release reports that one-year data from the DISCOVER post-market study that demonstrate excellent real-world results for the Direct Flow Medical TAVI valve.
Desolve bioresorbable scaffold continues to show low MACE rate at three years
Three-year data for Elixir Medical's Desolve novolimus-eluting bioresorbable scaffold indicate that the device is associated with a low overall major adverse cardiac events rate and sustained lumen gain.
European DISCHARGE trial will analyse whether CT can replace cardiac catheterisation in patients with suspected coronary disease
A DISCHARGE study currently examines whether CT or ICA are preferable for which patients with suspected coronary artery disease based on stable chest pain. The major goal of the trial is to determine whether CT helps to reduce myocardial infarction, stroke, and cardiovascular death.
Long-term results of RESPECT indicate that PFO closure may be effective at preventing recurrent stroke
The long-term study results from the RESPECT trial found that closing a patent foramen ovale (PFO) with an Amplatzer PFO Occluder was superior to medical management in the prevention of recurrent cryptogenic stroke in patients who previously had a cryptogenic stroke.
ABSORB III data reviewed
In this American College of Cardiology video, Peter Block and Dean Kereiakes discuss the results and implications of the ABSORB III study that was presented, by Kereiakes, at TCT 2015.
Medtronic CoreValve System ‘real-world’ US patient experience data replicates clinical outcomes
Medtronic has released the first outcomes data for its CoreValve Transcatheter Aortic Valve Replacement (TAVR) System using CoreValve data from The Society of Thoracic Surgeons and American College of Cardiology (STS/ACC) Transcatheter Valve Therapy (TVT) Registry. The everyday clinical experience of 6,160 patients has been shown to replicate the outcomes achieved in clinical trials.
Corindus Vascular Robotics and Mayo Clinic Launch Vascular Robotic Program to Research Physician Safety
Corindus Vascular Robotics has announced the formation of a joint robotic-assisted percutaneous coronary intervention (PCI) research and clinical program. This program utilises Corindus' CorPath System whilst protecting hospital employees from occupational radiation exposure and orthopedic stress and strain.
Stentys sirolimus-eluting stent confirms best-in-class performance at three years
Stentys has announced that the long-term performance of the siromilus-eluting Self-Apposing stent has been confirmed by three-year follow-up imaging data from the Stentys arm of the APPOSITION IV clinical. The data was presented by Robert-Jan van Geuns from Erasmus Medical Center in Rotterdam at TCT 2015.
Medtronic CoreValve Evolut R System demonstrates strong performance of recapturable and repositionable heart valve yielding low rates of mortality and stroke with excellent hemodynamics
Medtronic have announced new one-year data revealing exceptional clinical outcomes for transcatheter aortic valve replacement (TAVR) with their CoreValve Evolut R System. This system is the first and only next-generation recapturable, self-expanding valve available in the US market.
HeartFlow FFRCT Analysis can lower cost of care by up to 32% and improve quality of life for coronary artery disease
FFRCT Analysis technology from HeartFlow could lower the cost of evaluating patients with suspected coronary artery disease by as much as 32%, when compared to invasive coronary angiography. New data reveals the potential to use the technology to improve patient quality of life.
Medtronic CoreValve System shows low mortality and improved quality of life in new patient populations
Medtronic have presented new clinical data that shows positive clinical outcomes at one year for the CoreValve System in new populations including patients with a degenerated surgical bioprosthesis, end-stage renal disease and patients with low gradient aortic stenosis. Results from the three populations evaluated within the CoreValve US Expanded Use Study were presented at the Transcatheter Cardiovascular Therapeutics (TCT) symposium.
LIVE from TCT: No evidence of scaffold thrombosis with Biotronik’s magnesium scaffold at six months
According to results presented at TCT yesterday, the BIOSOLVE II study met is primary angiographic endpoint. Furthermore, the results also showed that there was no evidence of scaffold thrombosis with Biotronik's magnesium scaffold.
LIVE from TCT: Acceptable acute safety profile of Tryton side-branch stent confirmed
According to Tryton Medical, results from the Tryton Confirmatory Study confirm the acceptable acute safety profile of the Tryton side-branch stent for the treatment of coronary bifurcation lesions in vessels appropriate for a _2.5mm stent.
LIVE from TCT: Ranolazine does not reduce adverse events in patients with residual occlusions after PCI
The RIVER-PCI study, which was presented at TCT today, shows that ranolazine did not reduce the composite rate of ischemia-driven revascularisation or hospitalisation in patients with a history of chronic angina who had residual un-revascularised coronary artery disease after PCI.
LIVE from TCT: Promising results for novel drug-filled stent
Initial results, presented at TCT, from the Revelution trial indicate that Medtronic's drug-filled stent is associated with early vessel healing and controlled polymer-free drug elution.
Lariat surgical delivery device receives the CE mark
SentreHeart has received the CE Mark approval for its Lariat surgical left atrial appendage suture delivery device. The suture-based solution is designed for soft tissue closure, including the left atrial appendage closure.
Gary Lickovitch becomes vice president of sales and service at Corindus Vascular Robotics
Corindus Vascular Robotics has announced that it has named Gary Lickovitch as vice president of sales and service. He will report directly to Corindus' president and chief executive officer, David Handler.
Boston Scientific to present data for wide range of products at TCT
Key data presentations spanning Boston Scientific's broad interventional cardiology, structural heart and peripheral interventions portfolios will be shared at the TCT meeting (10-15 October, San Francisco, USA), the company has revealed.
Elixir will stream its first live case transmission of Desolve at TCT
Elixir Medical has announced that it will stream the first live transmission case demonstration of its Desolve Cx novolimus-eluting bioresorbable coronary scaffold system at TCT (11-15 October, San Francisco, USA).
Medtronic to unveil new clinical data at TCT
Medtronic has announced a preview of notable clinical studies that will be presented at the annual Transcatheter Cardiovascular Therapeutics (TCT) symposium (11-15 October, San Francisco, USA). The two-year results from the pivotal IN.PACT SFA clinical trial will be unveiled in a First Report Investigation presentation.
Cardinal Health completes acquisition of Cordis
Cardinal Health has announced that it has completed the acquisition of Johnson & Johnson’s Cordis business for $1.944 billion. Planning has been ongoing since the acquisition announcement in early March 2015, and the integration is off to a successful start with management teams in place worldwide.
Boston Scientific announces additional investment and right to acquire MValve Technologies
Boston Scientific has announced that it has closed on an additional round of financing with MValve Technologies, a developer of a percutaneous mitral valve replacement system, designed to work with the Boston Scientific Lotus valve.
New test could identify two-thirds of patients at very low risk of heart attack in the emergency department
Using a high sensitivity blood test, researchers have identified the optimal level of a protein called troponin that could rule out a diagnosis of heart attack for two-thirds of people attending the emergency department, according to new research.
Robotic PCI can now be used for radial access procedures in USA
The FDA has given 510(k) clearance to Corindus Vascular Robotics for its robotic-assisted CorPath System to be used during percutaneous coronary intervention (PCI) performed via radial access. The 510(k) clearance was based on results of a clinical trial, with an enrolment of 30 patients, that demonstrated 100% device and clinical success.
New randomised trial will compare sutureless valve to standard bioprostheses
Sorin has announced the initiation of PERSIST, which is the first international, prospective, post-market randomised multicentre trial evaluating the Perceval sutureless aortic valve compared to standard sutured bioprostheses in patients with aortic valve disease.
Positive preclinical data for trileaflet repair with CardioCel presented at EACTS
According to preclinical data presented at EACTS (3-7 October, Amsterdam, the Netherlands), CardioCel can be successfully used to perform trileaflet repair in a sheep model with no or minimal calcification detected.
Medtronic completes acquisition of Twelve
Medtronic has completed its acquisition of Twelve, which is a privately-held medical device company that is focused on the development of a transcatheter mitral valve implantation device. Medtronic says it looks forward to welcoming the Twelve team.
FDA issues notification about reduced leaflet motion with bioprosthetic aortic valves
The FDA has announced it is working with ACC, STS, and device manufacturers to design clinical studies to fully evaluate reduced leaflet motion for all types of bioprosthetic aortic valves. However, it says it believes such devices are reasonably safe and effective when used according to their FDA-approved indications.
Further studies needed to find effective treatment for reperfusion injury
In this commentary, Michael Gibson reviews the various approaches that have been evaluated for reperfusion injury and states why he believes further studies in this area are still needed.
50th patent for HeartFlow secured
The United States Patent and Trademark Office has issued to HeartFlow its 50th patent. The patent, "Method and System for Patient-Specific Modeling of Blood Flow" (US Patent No. 9,152,757), continues the expansion of companyt's patent portfolio based on its non-invasive HeartFlow FFRCT analysis.
First-in-man implant of MValve Technologies’ transcaheter mitral valve replacement system
MValve Technologies has revealed that the first human implantation of its catheter-based transapical mitral valve replacement system has taken place. The procedure was completed successfully at the University Clinic in Bonn, Germany.
First cohort of patients enrolled in FANTOM II clinical trial
The first cohort of 110 patients has been enrolled in the FANTOM II clinical trial. Reva Medical hopes to use the data, when available, from the study when it applies for the CE mark for its Fantom sirolimus-eluting bioresorbable scaffold.
Post-hoc analysis of Tryton side-branch stent trial published
A post-hoc analysis of the Tryton randomised controlled trial, now published in Catheterization and Cardiovascular Interventions, show that the Tryton side-branch stent is associated with reduced target vessel failure and improved side branch percent diameter stenosis in patients with vessels _2.25mm in diameter.
Venair collaborates on leading research project with Georgia Institute of Technology
Venair provides mold building and casting with silicone for physical model of left ventricle required for Georgia Institute's research. The mold built by Venair assists physical understanding of heart diseases and conditions.
Biosensors to commercialise BMX-J drug-eluting stent system in Japan
BMX-J is an original equipment manufacturer version of the Nobori drug-eluting stent, which consists of Biosensors’ unique drug-eluting stent that incorporates a biodegradable polymer and the company’s proprietary drug Biolimus A9.
St Jude Medical collaborates with Mended Little Hearts to produce The Mended Little HeartGuide
St Jude Medical and Mended Little Hearts have joined together to create The Mended Little HeartGuide-a comprehensive digital guidebook for parents and families of children with congenital heart conditions.
Myocardial injury after TAVI: What matters; what doesn’t?
CardioSource WorldNews talks to Lars Svensson about the clinical and functional outcomes of injuries to the myocardium after TAVI. The interview was conducted at ESC 2015.
World Heart Day focuses women’s heart health
Today is World Heart Day (29 September), which is organised by the World Heart Federation and aims to raise awareness of heart disease through education, advocacy and research, focuses on women's heart health. The British Heart Foundation reports women often wait longer than men before calling an ambulance after experiencing symptoms of a heart attack.
Occlutech receives the CE mark for its ventricle septal defect closure device
Occlutech now has CE mark approval for its muscular ventricle septal defect closure device-is a specifically designed implant indicated for the minimally invasive closure of muscular ventricle septal defects.
Primary endpoint met in HeartMate 3 CE mark trial
Thoratec has announced that the HeartMate 3 trial, which evaluated the HeartMate 3 left ventricular assist device, has met its primary endpoint-a comparison of six month survival to a performance goal derived from the INTERMACS registry.
RenalGuard significantly reduces acute kidney injury during TAVI
According to the results of PROTECT-TAVI, the use of RenalGuard during transcatheter aortic valve implantation (TAVI) procedures is associated with a significant reduction in in post-procedural acute kidney injury compared with standard treatment.
CE mark for new TAVI simulation software
FEops has received the CE mark for its transcatheter aortic valve implantation (TAVI) simulation software. The TAVIguide technology is designed to revolutionise preoperative planning for TAVI by providing insights into the interaction between a patient's native aortic root and the aortic valve.
More than 1000 patients have now been treated with Acurate Neo valve
Symetis has announced, in less than a year after the device was commercially launched, the Acurate neo/TF has been implanted in more than 1,000 patients. The company's other valve, Acurate TA system, has achieved a number two position in its segment of the European TAVI market.
Medinol completes enrolment of its multicentre trial of eDES
Medinol has announced that enrolment in its BIONICS trial, a global, prospective, randomised, multicentre, clinical trial designed to evaluate the safety and effectiveness of a new coronary stent system (eDES), has been completed.
CeloNova announces FDA approval to start a Cobra PzF coronary stent system randomised trial
This is a second major interventional cardiology trial for CeloNova in the USA, following the successful enrolment of the PzF SHIELD clinical trial earlier this year.
Miracor launches PiCSO impulse system in Asia
Miracor has announced the first treatments of patients with the PiCSO impulse system in the United Arab Emirates (UAE), marking the launch of this novel therapy in Asia in cooperation with Medical Technology.
CE mark for 27mm and 29mm of Portico TAVI valves
St Jude Medical has received the CE mark for its 27mm and 29mm Portico TAVI valves. The company says that it is now able to offer a full portfolio of Portico sizing options for physicians treating patients with aortic stenosis.
First-in-man cases with Mitra-Space presented
Cardiosolutions unveiled the first-in-human experience with its Mitra-Spacer system at PCR London Valves (20-22 September, Berlin, Germany). The novel system is intended to treat or bridge heart failure patients whose operative mortality risk for undergoing conventional open-heart surgery is deemed too high.
High definition IVUS used for the first time
Following the market release of the ACIST HDi intravascular ultrasound (IVUS), Scripps Clinic (La Jolla, USA) has become the first Cardiac Cath Lab in the world to use the new system. The system was used to optimise stent sizing and ensure proper stent expansion.
Open-label phase 1 study to evaluate off-the-shelf, biomaterial scaffold
Ventrix has initiated a phase 1, open-label study of its off-the-shelf, biomaterial scaffold (VentriGel). The scaffold is designed to facilitate the repair of cardiac tissue following myocardial infarction.
Incoming St Jude Medical president and chief executive officer announces executive leadership team
St Jude Medical has announced the new executive leadership team that incoming president and chief executive officer Michael T Rousseau has chosen to lead the organisation, effective from 1 January 2016.
First patient enrolled in CeloNova’s e-COBRA clinical registry
The French registry is evaluating the Cobra PzF coronary stent system in patients with heart disease.
Harpoon Medical enrols ten patients in Early Feasibility Study with 100% procedural success
The company has also received US$1.4m of a US$2m bridge round to accelerate its clinical programme.
Direct transfer of STEMI patients to cath lab reduces mortality
Lindsay L Anderson and others report that directly transferring patients with STEMI from the referring hospital to the cath lab-rather other departments (such as the emergency department)-of the receiving hospital is associated with significant reductions in time to reperfusion and mortality.
Sorin and Cyberonics announce appointment of LivaNova’s chief financial officer
Vivid Sehgal’s appointment as chief financial officer of LivaNova will be effective with the closing of the proposed merger of Sorin and Cyberonics.
Admedus to present CardioCel data At European cardio-thoracic conference
Admedus will present CardioCel data for the complete repair of aortic heart valves at the 29th European Association for Cardio-Thoracic Surgery Annual Meeting (EACTS) (3-7 October, Amsterdam, the Netherlands).
Medicure announces filing of sNDA for new Aggrastat indication
The supplemental New Drug Application seeks the addition of ST segment elevation myocardial infarction indication for Aggrastat.
Nipro to acquire Infraredx
The acquisition, which is subject to certain conditions, is expected to close in October 2015.
The OPTIDUAL trial
For EHJ today, Karl Swedberg moderates a discussion between Gerard Helft and Lars Wallentin about the OPTIDUAL study (which Helft presented at the ESC).
Rediscovering the transfemoral approach for structural heart interventions
Before we abandon "the good old femoral route" completely, the advent of structural heart disease interventions means that we still have a use for the approach. Flavio Ribichini explores the use of the transfemoral approach with these interventions.
Philips and Catharina Hospital sign technology agreement for largest cardiovascular centre in the Netherlands
More patients with complex cardiovascular diseases will soon be able to benefit from the latest facilities in the field of image-guided minimally invasive treatments at Catharina Hospital.
Analysis projects Carillon Mitral Contour System as a cost effective option for functional mitral regurgitation
Researchers analysed data derived from the TITAN clinical trial, which demonstrated significant clinical merits of the Carillon technology, and developed a comprehensive cost-effectiveness model that projects the costs of the technology and compares them to the benefits.
Thoratec announces start of SHIELD II US clinical trial
The SHIELD II US clinical trial will examine the use of the HeartMate PHP in patients undergoing a high-risk percutaneous coronary intervention (PCI) procedure.
FDA approves expanded Brilinta indication to include long-term use in patients with a history of heart attack
The US Food and Drug Administration (FDA) has approved AstraZenca's Brilinta (ticagrelor) tablets at a new 60mg dose to be used in patients with a history of heart attack beyond the first year.
Absorb is non-inferior to Xience in ABSORB-Japan
Results from the ABSORB-Japan study, which were presented at the 2015 European Society of Cardiology meeting, indicate that the bioresorbable vascular scaffold (Absorb, Abbott Vascular) is non-inferior to an everolimus-eluting stent with a permanent polymer (Xience, Abbott Vascular).
First patient enrolled in BIOFLOW-VI clinical study in China
The first patient has been enrolled in the BIOFLOW-VI clinical study in China. The aim of the study is further demonstrate the safety and efficacy of a hybrid drug-eluting stent (Orsiro, Biotronik) and support Chinese market approval.
Mitralign given the OK for early feasibility study of its percutaneous tricuspid valve system
The FDA has granted Mitralign investigational device exemption approval for the company to conduct an early feasibility study to examine its percutaneous tricuspid valve annuloplasty system. The SCOUT Study will take place in select centres in the USA.
Data show benefits of cangrelor in patients undergoing either radial or femoral approach
New data indicate that the intravenous antiplatelet agent cangrelor (Kengreal, The Medicines Company) is associated with significant reduction in ischaemic events compared with clopidogrel in patients undergoing PCI with either the femoral or the radial approach.
Javier Escaned
Javier Escaned (head of Section, Interventional Cardiology Unit, Hospital Clinico San Carlos, Madrid, Spain) is a co-course director of EuroPCR (17-20 May, Paris, France).
FFRCT significantly reduces the number of invasive tests with no sign of obstructive disease
The PLATFORM study, which was presented at the ESC, indicates that use of FFRCT is associated with a significant reduction in the number of invasive tests that do not find evidence of obstructive coronary artery disease.
HeartWare International to acquire Valtech Cardio
Valtech Cardio specialises in the development of innovative surgical and transcatheter valve repair and replacement devices for the treatment of mitral valve regurgitation and tricuspid valve regurgitation.
LIVE from the ESC: Five-year FAME data further confirms benefits of FFR-guided PCI
The five-year results of the FAME study, which were presented at the ESC yesterday, have confirmed that FFR-guided PCI has sustained, long-term benefits compared with angiography. They show that FFR-guided PCI can contribute to reductions in all-cause mortality, cardiac mortality and an overall use of healthcare resources.
LIVE from ESC: No benefit with aldosterone antagonists in acute myocardial infarction
In the ALBATROSS study, which was presented at the ESC Congress this morning, adding aldosterone to standard therapy in patients with acute myocardial infarction was not associated with a significant reduction in the primary outcome (which included death, resuscitated cardiac arrest, and significant ventricular arrhythmia)
Philips to showcase integrated cardiology solutions ESC Congress 2015
Royal Philips has announced its presence at ESC Congress 2015, where the company is showcasing its latest cardiology solutions, including Heart ModelA.I., EchoNavigator and IntelliSpace Cardiovascular
Risk of all-cause mortality with post-discharge bleeding after PCI is greater than risk with post-discharge myocardial infarction
Philippe G̩n̩reux and others report in the Journal of the American College of Cardiology that data from ADAPT-DES indicate that the risk of all-cause mortality that is associated with post-discharge bleeding after PCI is 2.6-fold greater than the risk of all-cause mortality that is associated with post-discharge myocardial infarction.
FDA approval for evolocumab
The FDA has approved evolocumab (Repatha, Amgen), which is a new cholesterol-lowering medication that inhibits proprotein convertase subtilisin/kexin type 9 (PCSK9)-a protein that reduces the liver’s ability to remove low-density lipoprotein cholesterol (LDL-C).
Benefits and challenges of secondary prevention after myocardial infarction
This European Society of Cardiology (ESC) video explores the role of comprehensive rehabilitation programmes for the secondary prevention of myocardial infarction.
ESC “very excited” to be in London
The president of the ESC, Fausto Pinto, claims that the society is "very excited" to be holding its annual congress in London (UK) this week, stating that the city "has a fantastic reputation for welcoming people from all around the world from international sporting competitions to ground-breaking educational and medical conferences."
The Medicines Company announces participation at 2015 European Society of Cardiology Congress
The Medicines Company will present results of recent clinical studies from its cardiovascular product portfolio at the 2015 European Society of Cardiology (ESC) Congress (29 August-2 September, London, UK).
Direct Flow Medical appoints Mary Edwards as vice president of regulatory and clinical affairs
Edwards will oversee all global clinical and regulatory efforts including the company's US SALUS pivotal trial studying the Direct Flow Medical Transcatheter Aortic Valve System, as well as the DISCOVER post-market European study.
SentreHEART elects Richard Ferrari to board of directors
Ferrari is currently the managing director and co-founder of De Novo Ventures and brings a wealth of strategic and operational knowledge SentreHEART's board.
Colibri Heart Valve receives patent for controlled release of percutaneous heart valve device
Colibri Heart Valve has received an issue notification from the US Patent and Trademark Office regarding the forthcoming granting of a US Patent titled, "Percutaneous replacement heart valve and a delivery and implantation system."
Medtronic to buy company that is developing a transcatheter mitral valve implantation device
Medtronic has announced that it has signed a definitive agreement to acquire the company Twelve, which is focused on the development of a transcatheter mitral valve implantation (TMVI) device.
2015 TCT late-breaking trials announced
The Cardiovascular Research Foundation (CRF) has announced the late-breaking trials and first report investigations that will be presented at this years' Transcatheter Cardiovascular Therapeutics (TCT) 2015 scientific symposium.
Clinical trial launched to test if coronary stent patients can avoid taking aspirin
The international clinical trial TWILIGHT will test the safety and effectiveness of treating coronary stent patients with the anti-clotting medication ticagrelor instead of combining it with aspirin.
Cyberonics and Sorin provide merger update
Cyberonics has set a date for a special meeting of its stockholders to consider and vote on the transaction and certain other related matters on 22 September.
“We all need the strength” to challenge unacceptable behaviour
The editors of Annals of Internal Medicine have called for doctors to confront colleagues who act in a disrespectful manner towards patients after an anonymous essay in the journal highlighted incidences in which unprofessional behaviour was condoned.
Heart attack patients without obstructive coronary artery disease are at high risk of residual angina
This is the first study to focus on the burden of residual angina after an initial heart attack and re-hospitalisations in patients without versus with obstructive coronary artery disease.
Corindus Vascular Robotics partners with Unfors RaySafe to help reduce radiation exposure in the cath lab
Corindus Vascular Robotics and Unfors RaySafe have announced a distribution agreement to allow Corindus to offer the RaySafe i2 real-time radiation dose monitoring system in conjunction with its CorPath system.
Bifurcation lesions: When a two-stent technique is needed
The advent of second-generation drug-eluting stents and the associated improved safety and efficacy has meant that the risks of using two stents for bifurcation lesions has significantly decreased. Maciej Lesiak explores when a two-stent strategy should be used.
Abiomed submits supplements to expand Impella 2.5 FDA pre-market approval
Abiomed has submitted US Food and Drug Administration pre-market approval supplemental submissions to expand Impella 2.5 pre-market approval to the entire Impella family of devices (Impella 2.5, Impella CP and Impella 5.0/LD).
US Endovascular and BrosMed enter into exclusive agreement to distribute coronary angioplasty catheters
US Endovascular and BrosMed have entered into an exclusive agreement to distribute Artimes semi-compliant and Apollo non-compliant coronary angioplasty catheters.
Interventional cardiology devices market to be worth US$25.16bn by 2020
Increasing prevalence of cardiovascular diseases coupled with adoption of minimally invasive surgeries is expected to boost the interventional cardiology device market over the forecast period.
Medtronic issues recall for EnVeo R loading system
Medtronic has issued a Class 1 recall of its EnVeo R loading system following eight reports of the presence of particulates, which could potentially lead to blockages in the bloodstream.
A team approach to cardiac arrests after cardiac surgery
In this video for the Cardiothoracic Surgery Network, Jill Ley leads an expert panel discussion about managing patients with cardiac arrest after cardiac surgery. This video was filmed at the Society of Thoracic Surgeons' annual meeting.
Successful testosterone replacement therapy reduces the risk of cardiovascular events and mortality in men
A study indicates that normalisation of total testosterone levels with testosterone replacement therapy is associated with a significant reduction in the risk of myocardial infarction, stroke and all-cause mortality compared with non-normalisation with testosterone replacement therapy and no therapy in men with low testosterone.
FDA issues safety warning about left ventricular assist devices
The FDA has issued a safety alert telling healthcare professionals, patients, and carers about potential serious adverse effects with left ventricular assist devices. The alert relates to Thoratec's HeartMate II device and HeartWare' HVAD.
Tryton Medical completes enrolment in the Extended Access Registry for its Tryton side branch stent
The Tryton registry is designed to confirm the results from Tryton's pivotal Investigational Device Exemption (IDE) trial, and has successfully enrolled 133 patients from Europe and the USA.
Nitinol Devices & Components acquires Interface Catheter Solutions to create Confluent Medical Technologies
A press release says that the combined company will leverage the technical expertise of both NDC and Interface in highly precise and demanding medical devices.
Getting to grips with social media
Pascal Meier believes that social media is the logical next step from the internet-just as the internet has revolutionised connectivity, social media is revolutionising communication. In this commentary, he explains why social media presents a great opportunity for interventional cardiologists.
Bellerophon Therapeutics announces results from PRESERVATION I clinical trial for Bioabsorbable Cardiac Matrix
The results showed no statistically significant treatment differences between patients treated with the Bioabsorbable Cardiac Matrix and patients treated with placebo for both the primary and the secondary endpoints.
Abbott Vascular expands its structural heart portfolio with new mitral valve technologies
Abbott Vascular has announced it has entered into an agreement to purchase Tendyne Holdings (focused on developing minimally invasive mitral valve replacement therapies) and secured an option to purchase Cephea Valve Technologies (which is developing a catheter-based mitral valve replacement therapy).
Social media for interventional cardiologists: rewards and risks in daily practice
In this PCR video, Simon Walsh (UK) interviews Pascal Meier (Switzerland) about the potential of social media to contribute to overall education and knowledge of interventional cardiologists, and to actually improve the clinical management of patients as it facilitates the exchange of knowledge. However, the use of social media is not without risk.
CorPath system to be featured for the first time in Japan
In addition to allowing cardiologists to advance stents and guidewires with precision using digital controls, the system enables physicians to perform procedures while seated in a lead-lined interventional cockpit protected from radiation exposure.
First participants enrolled in dabigatran study of AF patients undergoing PCI
Boehringer Ingelheim has announced that the first US patients have been enrolled in its international clinical trial-RE-DUAL PCI, which is evaluating the efficacy and safety of dabigatran in patients with non-valvular atrial fibrillation who have undergone percutaneous coronary intervention (PCI).
Natec Medical receives FDA approval for Filao NC PTCA balloon catheter
Natec Medical has received 510k approval from US Food and Drug Administration (FDA) for its Filao NC PTCA balloon catheter, Natec's third device to obtain FDA approval.
Survey shows need for greater consistency in “real world” observational research
Continuum Clinical's ninth annual survey reveals that drug and device companies understand the value of real world studies, but continue to struggle with strategic, operational and organisational hurdles.
New stem cell delivery method shows promise for treating end stage heart failure
A new clinical trial to test how a high dose of stem cells delivered via a method called retrograde coronary sinus infusion affects end stage heart failure patients is showing promising results.
Sunshine Heart provides update on COUNTER HF US study for C-Pulse heart assist system
COUNTER HF is a prospective, randomised, multicentre, controlled study evaluating the safety and efficacy of the C-Pulse system for the treatment of NYHA Class III and ambulatory Class IV heart failure.
Micell Technologies announces first patient enrolled in registration trial of MiStent SES in China
Micell Technologies has begun enrolling patients in DESSOLVE C: a prospective, single-blind, multicentre, randomised, controlled clinical trial to demonstrate the efficacy and safety of its MiStent SES sirolimus-eluting absorbable polymer coronary stent system.
Hospira announces US launch of generic bivalirudin for injection
Hospira’s bivalirudin for injection is available in a single-dose flip-top vial, which matches the current branded offering available.
Global bioabsorbable scaffolds market will be worth more than US$2bn by 2021
The growth will be driven primarily by new product approvals and a paradigm shift in the clinical treatment of vascular diseases towards minimally-invasive procedures, says research and consulting firm GlobalData.
EUROMAX substudy suggests transradial approach does not improve clinical outcomes
Contrary to the results of previous studies, according to a substudy of the EUROMAX trial, the transradial approach for coronary interventions is not associated with an improvement in clinical outcomes compared with the transfemoral approach.
Phil Mui joins HeartFlow product and engineering team
A former technology executive of Google and Acxiom, Mui will be responsible for guiding the scalable development of HeartFlow's technology platform.
Definitive agreement between St Jude Medical and Thoratec announced
St Jude Medical and Thoratec have announced that the Boards of Directors of both companies have unanimously approved a definitive agreement under which St Jude Medical will acquire all of the outstanding shares of Thoratec for $63.50 per share in a cash transaction valued at approximately $3.4 billion, net of cash acquired.
CE mark and FDA approval for new TAVI guidewire
Boston Scientific has received the CE Mark and FDA clearance for its Safari2 pre-shaped guidewire-a new and enhanced version of the Safari guidewire-for introducing and placing interventional devices within the heart (including those used in transcatheter aortic valve implantation).
A NOTION of things to come in TAVI
CardioSource World News talks with Hans Gustav HÌürsted Thyregod, about the NOTION study - the first all-comers trial to randomise low-risk patients to transcatheter aortic valve implantation (TAVI) or surgical aortic valve replacement.
Strut coverage: Optical coherence tomography and new stent technologies
Tim Kinnaird explores the risk of stent thrombosis with current technologies, how new technologies may be able to reduce this risk, and how OCT can help to assess this risk.
Boston Scientific launches bioresorbable scaffold trial
Boston Scientific has initiated a study to evaluate its fully resorbable scaffold technology. FAST (Fully absorbable scaffold feasibility study) is a prospective, single-arm study designed to assess the safety and performance of this next-generation scaffold.
OrbusNeich announces enrolment of first patient in China Recovery Study
The China Recovery Study is designed to evaluate the performance of OrbusNeich’s Combo stent compared with the Nano stent (Lepu Medical Technology).
HeartWare announces first human implants of the MVAD System in CE mark trial
The MVAD Pump is a heart pump that supports a wide range of flows to enable circulatory support for patients with advanced heart failure.
Vital’s CT myocardial perfusion makes its US debut at SCCT 2015
Vital Images’ 510(k) FDA-cleared computed tomography (CT) myocardial perfusion application is debuting at the 10th Annual Scientific Meeting of the Society of Cardiovascular Computed Tomography (SCCT; 16-19 July, Las Vegas).
US hospital is first hospital in world to use 4D ultrasound software to image heart
Aurora St Luke's Medical Center (Milwaukee, USA), a national leader in heart and vascular care, has become the first hospital in the world to use 4D ultrasound software, designed by GE Healthcare, to evaluate heart conditions.
Distal Access sells rights to SPNIR platform to Merit Medical
Distal Access has announced that it has sold the rights to its SPINR platform for peripheral, coronary, and endoscopic use to Merit Medical System's NRI Limited. The SPINR high-performance guidewire controller is designed to improve guidewire control, torque, and performance.
Men believe “long working hours” stop women from being interventional cardiologists
According to a new survey published in EuroIntervention, men state that the long working hours and the need to be on call that is associated with being an interventional cardiologist are the key reasons why so few women choose the subspecialty.
Enrolment in COAST study of orbital atherectomy system completed
Cardiovascular Systems has completed enrolment in COAST (Coronary orbital atherectomy system trial), which is designed to assess the safety and efficacy, as well as economic outcomes, of the company's new micro crown Orbital Atherectomy System (OAS).
Sorin launch latest generation of its healing and cooling system
Sorin has announced the global launch of its latest generation of heating and cooling system. The company reports that the FlexTherm-the newest addition to Sorin Heartlink System-is fully integrated with Sorin S5 industry-leading heart-lung machine.
Edwards Lifesciences enters into agreement to buy CardiAQ
Edwards Lifesciences has announced that it has agreed to acquire CardiAQ Valve Technologies, a privately held company and developer of a transcatheter mitral valve implantation (TMVI) system.
HeartMate PHP now has the CE mark
Thoratec has announced that its HeartMate PHP (Percutaneous Heart Pump) has received CE mark approval, permitting sale in the EU and other international countries. The approval was based on data from the first 30 patients enrolled in the HeartMate PHP SHIELD I CE mark trial.
FDA approves Promus Element Plus and Promus Premier coronary stent systems
The FDA has announced it has approved Boston Scientific's Promus Element Plus and Promus Premier everolimus-eluting, platinum chromium coronary stent systems (monorail and over-the-wire) systems.
US generic version of bivalirudin authorised
The Medicines Company has authorised, in an agreement with Sandoz, the distribution of an authorised generic of bivalirudin (Angiomax) for injection in the USA. The company states that the agreement will help to ensure that bivalirudin remains a high quality product in this market.
FDA strengthen warnings about risk of myocardial infarction with NSAIDS
The FDA has strengthened its existing label warning that non-aspirin nonsteroidal anti-inflammatory drugs (NSAIDs) increase the chance of myocardial infarction (or stroke). This follows, according to the agency, a comprehensive review of new safety information.
Nearly one third of PCI deaths are related to acute kidney injury
A new study indicates that nearly one third of in-hospital deaths after PCI can be attributed to acute kidney injury. It also shows that preventing nine cases of acute kidney injury could potentially prevent one death.
Early discharge after TAVI is feasible in selected patients
A retrospective analysis, published in Heart, indicates that early discharge after transfemoral TAVI in selected patients does not increase the risk of death at 30 days compared with later discharge.
Invisible impact: The risk of ionising radiation on cath lab staff
This ORSIF video tells the story of one of the world's most prominent cardiovascular surgeons, Edward Diethrich, and the career-altering health issues he has faced as a result of chronic, low-level exposure to ionising radiation through his work.
CardioCel shows no evidence of calcification after seven years in long-term follow-up to phase II trial
Admedus has announced positive long-term data from the CardioCel phase II clinical trial assessing the efficacy and safety of the company's proprietary bio-scaffold which has been implanted to repair congenital heart disease defects.
Thoratec announces first HeartMate 3 implant in European less invasive surgical trial
The implant begins a European study to evaluate outcomes and complications using less invasive surgical placement through hemisternotomy and left thoracotomy techniques.
Trend towards better outcomes with sutureless valve for high-risk patients
A study indicates that surgical aortic valve replacement using the right anterior minithoracomy approach and a sutureless valve is associated with a trend towards better postoperative outcomes than those with TAVI
Oxford University Press launches European Heart Journal-Quality of Care and Clinical Outcomes
EHJQCCO is the eleventh European Society of Cardiology journal and focuses on the quality of care affecting cardiovascular outcomes at hospital, regional, national, and international level.
Will the DAPT trial change my practice? The Asian perspective
Chee Tang Chin presents his group's paper on the Asian perspective of the DAPT trial published in AsiaIntervention and also asks Thomas Cuisset for his projection for DAPT therapy in Europe.
The second generation of drug-eluting stents with biodegradable polymers may have potential advantages
Drug-eluting stents with biodegradable polymers have theoretical advantages over drug-eluting stents with permanent polymers-even second-generation drug-eluting stents.
Bureaucracy leads to delays in patients accessing cardiovascular devices
Patients are experiencing significant delays in access to approved cardiovascular devices due to bureaucratic inefficiencies, a Devices White Paper from the Cardiovascular Round Table (CRT) has found.
Miracor Medical Systems appoints international scientific advisory council
Miracor Medical Systems has appointed an international scientific advisory council consisting of leading clinicians to support the further clinical and technological progress of its PiCSO acute myocardial infarction impulse system.
Cell Therapy reports 100% MACE-free survival after two years in the Heartcel clinical trial
At the International Society of Stem Cell Research Annual Conference (24-27 June, Stockholm, Sweden), the company also reported a 70% quality of life improvement measured by the MLHF questionnaire.
Cardiac Dimensions announces initial enrolments in REDUCE FMR clinical trial
The study is the first blinded, randomised clinical trial evaluating a mitral valve repair device in patients with functional mitral regurgitation.
FDA approves Medtronic’s CoreValve Evolut R
Medtronic has announced that the FDA has approved its recapturable, self-expanding CoreValve Evolut R TAVI system.
Tailored patient education may help to improve adherence to medications
Robin Mathews (Duke Clinical Research Institute, Duke University Medical Centre, Durham, USA) and others report, in Circulation: Cardiovascular Quality Outcomes, that tailored patient education may represent an opportunity to optimise patient adherence.
The importance of the PLATINUM DIVERSITY trial
The PLATINUM DIVERSITY trial was initiated last year with the aim of evaluating the clinical outcomes of an everolimus-eluting stent (Promus Premier, Boston Scientific) in patient populations that have been traditionally under-served by clinical trials (eg. women and minorities).
Admedus granted Cardiocel approval in Singapore
The application was based on the submission for US Food and Drug Administration (FDA) approval, which includes indications for use in cardiovascular repairs, suture buttressing and vascular and vessel repairs in both adults and children.
Learn About the “After the Stent” Campaign
Jeffrey Cavendish, interventionalist and member of The Society for Cardiovascular Angiography and Interventions (SCAI) Foundation, and Donnette Smith, executive vice president of Mended Hearts, discuss the "After the Stent" campaign.
CardiAQ reports first-in-human percutaneous implantation with its second generation transcatheter bioprosthetic mitral heart valve
The second-generation bioprosthetic mitral heart valve was implanted into a 72-year-old male suffering from severe mitral regurgitation (MR 4+) with multiple co-morbidities and ineligible for alternate treatment modalities.
Sapien 3 now approved for use in the USA
Edwards Lifesciences has announced that the FDA has approved its latest transcatheter aortic valve implantation (TAVI) system-Sapien 3-for the treatment of high-risk patients suffering from severe, symptomatic aortic stenosis.
First US patient enrolled in BIOFLOW-V study of Orsiro
The first US patient-Biotronik has revealed-has been enrolled in the BIOFLOW-V clinical study of the Orsiro hybrid drug-eluting stent. The aim of the study is further demonstrate the safety and effectiveness of the device.
Vascular Solutions introduces two new versions of Turnpike catheters
All three versions of the Turnpike are over-the-wire catheters with an advanced shaft design that provides superior tracking and advancement over a 0.014" guidewire for use in complex coronary and peripheral interventions.
CorPath system robotically deploys bioresorbable vascular scaffold during a live PCI broadcast at 2015 C3 Conference
Delegates watched Arif Al Nooryani perform the triple-vessel disease case robotically in real-time by deploying bioresorbable stents, wiring the lesion and placing the stents using the CorPath system.
Randomised controlled trials must be simplified to sustain innovation
The call comes from the Cardiovascular Round Table, an independent forum established by the European Society of Cardiology and comprised of cardiologists and representatives of the pharmaceutical, device and equipment industries.
Opsens receives clearance to sell FFR products in the USA
Opsens has received 510(k) clearance from the FDA for its OptoWire and OptoMonitor, its products, which have been developed to measure fractional flow reserve (FFR).
Philips launches anatomically intelligent quantification tool for cardiac ultrasound imaging
The tool was unveiled on the EPIQ 7 ultrasound system, Philips’ first ultrasound with anatomic intelligence capabilities, during the American Society of Echocardiography annual meeting (12-16 June, Boston, USA).
Visualising calcified coronary arteries may be wake-up call to change lifestyle
Looking at images of their own calcified coronary arteries may be a wake-up call for patients with newly diagnosed coronary artery disease to change their lifestyles, reveals new research.
Poor sleep associated with increased risk of heart attack and stroke
Poor sleep should be considered a risk factor for cardiovascular disease along with smoking, lack of exercise and poor diet.
New survey will explore international barriers to adopting TAVI
Following its 2012 survey of the barriers that prevent patients in Europe from having access to transcatheter aortic valve implantation (TAVI), BIBA MedTech has launched a new survey to gain insight into the barriers that stop patients outside of Europe from having this procedure.
Comparable outcomes between PCI and surgery in diabetic patients with acute coronary syndromes and multivessel disease
The ACUITY study indicates that there are no significant differences in rates of myocardial infarction or death between diabetic patients with acute coronary syndromes and multivessel disease undergoing PCI and those undergoing CABG.
The current status of transcatheter pulmonary valve replacement
The Melody transcatheter pulmonary valve (Medtronic), which received the CE mark in 2006 and FDA humanitarian device exemption approval in 2010, was recently granted FDA premarket approval. In this commentary, Darren P Berman explores the mid- and long-term data for the device
Deepak L Bhatt
Deepak L Bhatt (Executive director of Interventional Cardiovascular Programs, Brigham and Women's Hospital Heart & Vascular Center, Boston, USA) was the co-principal investigator of SYMPLICITY HTN-3, which he believes highlighted the value of sham-controlled trials.
TCTAP 2015 Wrap-up Interview “CABG vs. PCI for LM or 3VD”
TCTAP 2015 Wrap-up Interview that explores the role of CABG vs. PCI for left main or three vessel disease. The moderator is David Paul Taggart with interviewees Seung-Jung Park and Patrick W. Serruys
Biotectix announces licensing agreement with Acutus Medical for the use of Amplicoat on cardiac catheters
Amplicoat is a coating designed to enhance communication at the interface between human tissue and a medical device's electrode, and enable higher signal fidelity, reduced power requirements, and device electrode miniaturisation.
FDA expands label indication for PleuraFlow to reduce retained blood
The FDA's expanded indications for use also allow the PleuraFlow system to be used in all cardiothoracic surgery and chest trauma procedures for adult and paediatric patients.
Early healing profile established for BioFreedom
Results in the first twelve months demonstrate rapid strut coverage, suggesting an early healing profile for patients using BioFreedom.
Article highlights effects of orbital atherectomy and rotational atherectomy in treating heavily calcified coronary lesions
Study finds significant tissue modification associated with orbital atherectomy led to better stent apposition and expansion.
Isaac George named co-principal investigator of pivotal trial of Direct Flow Medical’s Transcatheter Heart Valve System
George joins co-principal investigator Murat Tuzcu, vice chairman of the department of cardiology for the Cleveland Clinic, in leading the trial.
New data add to evidence base for FFR
New results from two clinical studies, both of which were presented at EuroPCR (19-22 May, Paris, France) provide further support to the use of St Jude Medical's fractional flow reserve (FFR) technology to optimise percutaneous coronary intervention (PCI) procedures.
Merit Medical Systems launches the Prelude Snap splittable sheath introducer
Merit Medical Systems has launched the Prelude Snap splittable sheath introducer, the design of which is based directly on physician feedback.
Jenavalve appoints Victoria E Carr-Brendel as chief executive officer
Carr-Brendel replaces Jan Keltjens, who served as interim chief executive officer since January 2015. Keltjens will remain chairman of the JenaValve board and will support Carr-Brendel during a transition period.
Award for artificial mitral valve pioneer
Albert Starr has received the 2015 Institut de France's Grand Prix Scientifique for work that led to the world's first successful artificial mitral valve implant. As a result of this success, Miles Lowell Edwards incorporated Edwards Laboratories to manufacture and market the Starr-Edwards valve
Philips launches “first of its kind” on-demand ultrasound service
Philips has today announced the launch of "Ultrasound on Demand" in the UK, which it claims is the world's first on-demand ultrasound solution. The system aims to provide clinicians with access to the best technology at an affordable monthly fee, offering extensive functionality as well as meeting the ever-changing needs of busy hospital departments
Toshiba introduces software upgrades for 3T MRI system
Toshiba says that the upgrades improve image quality and workflow "so those in both the clinical and research settings have access to the highest levels of performance and information to provide the best possible care."
New 7T MRI research system ready for future clinical use
The 7T Magnetom Terra is the first fully designed and manufactured by Siemens, with a new 7T magnet in its core, and comes with the lightest actively shielded 7T whole body magnet.
New trial does not support earlier findings that complete revascularisation improves outcomes
Results from the PRAGUE 13 trial indicate that there are no significant differences in outcomes between staged percutaneous coronary intervention (PCI) and culprit-only PCI in patients with multivessel disease; these results differ from those of earlier studies.
Non-invasive FFRCT could transform management of coronary artery disease
Daniel Simon reviews a new system that simulates fractional flow reserve (FFR) measurements using computed tomography (FFRCT, HeartFlow). He explains why he believes this system, which recently received FDA approval, has the potential to reduce the number of unnecessary coronary tests that are performed.
European Bifurcation Club: 10-year anniversary consensus document
European Bifurcation Club 10-year anniversary consensus document was the most downloaded EuroIntervention paper from the last 12 months. Lead Author Jens Flensted Lassen provides a brief summary of the main findings of this EBC document particularly in reference to the one vs two stent dilemma in coronary bifurcation treatment.
Study shows larger capacity intra-aortic balloon pumps to have greater haemodynamic effects
The retrospective study examined demographic, haemodynamic, and laboratory data in 26 consecutive subjects treated with a 50cc IABP and compared them with 26 patients receiving a 40cc IABP between 2012 and 2013.
Thoratec receives conditional FDA approval for the SHILED II US clinical trial for HeartMate PHP
The clinical trial will examine the use of Thoratec's HeartMate PHP acute catheter-based heart pump in patients undergoing a high-risk percutaneous coronary intervention.
Abbott announces CE mark for advancement of Absorb stent system for heart disease
The Absorb GT1 combines a fully dissolving stent with a next-generation delivery catheter to help doctors treat people with heart disease.
InspireMD CGuard reports positive results at EuroPCR 2015
Results from the PARADIGM trial indicated that the CGuard system is appropriate for use in an all-comer carotid revascularisation population and is associated with favourable angiographic and clinical outcomes.
REVA releases initial clinical results for Fantom scaffold
Acute performance was demonstrated with 100% technical and procedural success and no reported major adverse cardiac events, with no incidence of ischaemic target lesion revascularisation, myocardial infarction or stent thrombosis.
Positive data for Mitralign percutaneous annuloplasty system presented at EuroPCR
According to six-month data presented at EuroPCR (19-22 May, Paris, France), the Mitralign percutaneous annuloplasty system (MPAS) is associated with significantly improved valve function.
Arterial Remodeling Technologies and Terumo show promising pre-clinical data for drug-eluting bioresorbable scaffold
The findings were announced at the Arterial Remodeling Technologies symposium at the EuroPCR 2015 meeting (19-22 May, Paris, France).
EuroPCR 2015 Orsiro hybrid drug-eluting stent data highlights safety and efficacy in high-risk patients
Experts at the Biotronik-sponsored symposium discussed Orsiro's performance in difficult cases as well as coming developments in percutaneous coronary intervention.
Elixir Medical announces three-year imaging results for the DESolve scaffold system
Stefan Verheye, co-principal investigator of the DESolve Nx trial, presented imaging results of multiple cases from a subset of 14 patients scheduled to be followed up with angiography and OCT at three years as part of the study.
On-X Life to launch Chord-X mitral valve chordal repair system
The On-X LTI Chord-X used during mitral valve repair is produced with On-X LTI’s proprietary ePTFE suture and has been designed to provide surgeons new tools to help simplify the procedure.
Long-term safety and efficacy of BioMatrix drug-eluting stent family confirmed in large international registry
The final long-term results of the e-BioMatrix registry, which was presented at EuroPCR 2015 (19-22 May, Paris, France) by David Hildick-Smith (Sussex Cardiac Centre, Brighton, UK), have confirmed that the family of BioMatrix drug-eluting stents are safe and efficacious
Biotronik announces successful first implantations of resheathable transcatheter aortic valve
Implantations in aortic stenosis patients at the University Heart Center Hamburg-Eppendorf (UKE), Germany, confirm safety at 30 days.
Data presented at EuroPCR 2015 demonstrate strong performance of Synergy over four years
Additional data from EVOLVE II pivotal trial demonstrate safety and performance in patients with diabetes at one year.
Use of CoreValve is as safe and effective as surgery in low risk patients at two years
Presented as a late-breaking clinical trial at EuroPCR, new results from the NOTION trial showed that TAVI with CoreValve is as safe and effective as surgery in patients that are at a low- or intermediate-risk for surgery.
Direct Flow Medical reports two-year data for its Transcatheter Aortic Heart Valve System
Direct Flow Medical has announced positive two year data from the DISCOVER CE mark trial studying its Transcatheter Aortic Valve System at EuroPCR 2915 (19-22 May, Paris, France).
Significant differences in achieving risk factor targets between women and men following a heart attack
These gender differences are reflected in the rate of risk factor control, which was lower in women, and in the rate of hospital readmission for a further heart attack, which was higher in women than in men.
OrbusNeich announces initiation of HARMONEE stent study in USA
The registration study will enrol 572 patients at up to 50 study locations in Japan and the USA.
Availability of HeartFlow FFRCT Analysis may change the management of patients with coronary artery disease
Results of the FFRCT RIPCORD study were presented at EuroPCR 2015 (19-22 May, Paris, France) by Nick Curzen of the University Hospital Southampton, UK.
Post-market study evaluating the Lotus valve system demonstrates low paravalvular aortic regurgitation rates
Thirty-day results for the first 250 patients in the RESPOND post-market study were presented at EuroPCR 2015 by Nicolas M Van Mieghem, Erasmus Medical Center in Rotterdam, the Netherlands.
First clinical experience with Stenty’s Xposition shows 100% implantation success
The findings were presented at the EuroPCR 2015 conference (19-22 May, Paris, France) and were also published in the online edition of EuroIntervention.
Post-market study of Direct Flow Medical’s aortic heart valve system demonstrates positive outcomes at 30 days
The data were presented at EuroPCR 2015 (19-22 May, Paris, France) by Christoph C K Naber, from the Contilia Heart Centre in Essen, Germany.
Edwards pauses enrolment in early stage mitral programme
A company press release states that the pause in enrolment is due to observed evidence of valve thrombosis that it believes warrants additional investigation.
SERVE-HF sleep apnoea trial fails to meet primary endpoint
Trial designed to assess whether Adaptive Servo-Ventilation therapy could reduce mortality and morbidity in moderate to severe predominant central sleep apnoea patients with symptomatic chronic heart failure in addition to optimised medical care
Institutional factors play role in cardiac rehab referral rates after angioplasty
Hospitals in the Midwest of the USA were more likely than others to refer patients for guideline-recommended cardiac rehabilitation following angioplasty, possibly because more rehab programs are available in the region.
Harpoon Medical enrols patients in study of its repair system for off-pump, minimally invasive treatment of mitral valve regurgitation
The initial first-in-human procedures were performed at the Department of Cardiovascular Surgery and Transplantology, Jagiellonian University John Paul II Hospital, Poland.
Two-year ORBIT II coronary data and long-term economic data presented at SCAI 2015
The study examined the use of Cardiovascular Systems’ Diamondback 360 device in treating patients with de novo severely calcified coronary lesions.
Tips and tricks for achieving optimum results with a drug-coated balloon
With drug-coated balloons, we may have been able to deliver a device to the lesion, but we were not always sure how much drug we actually delivered. Fortunately, because of this problem, there have been huge improvements to drug-coated balloon technology. However, certain "tricks" are needed to achieve optimum results with drug-coated balloons.
Pie Medical Imaging introduces its newest 3mensio structural heart analysis software
The new release includes an optimised mitral workflow and a new septal crossing workflow for planning of mitral valve procedures to determine the appropriate access route based on computed tomography images.
LRP study enrolls 1,000 patients
Infraredx has announced the enrollment of 1,000 patients in its Lipid-Rich Plaque (LRP) study. The study is a prospective, multicentre clinical trial designed to identify a correlation between lipid-rich plaques detected by the company's TVC Imaging System and the occurrence of a cardiac event within two years.tvc
Jean Boulle Group announces first US clinical trial implant of Tendyne transcatheter mitral valve
Boulle Medtech, a Jean Boulle Group medical technology company and the founding investor of Tendyne Holdings, has announced that the Tendyne transcatheter mitral valve implant was successfully implanted in the first patient in the USA.
ResMed launches AirCurve 10 CS PaceWave for central sleep apnoea
ResMed has announced the launch of its ResMed Air Solutions range, led by the intelligent, cloud-enabled AirCurve 10 CS PaceWave with AirView system. This is the first device to combine remote monitoring with ResMed's iPaceWave minute ventilation (MV)-adaptive-servo-ventilation (ASV) therapy.
Medtronic initiates study evaluating potential of combination of pulmonary vein isolation and renal denervation for atrial fibrillation
Medtronic has announced the start of a clinical study using Medtronic technologies to determine whether paroxysmal and persistent atrial fibrillation can be treated with a combination of two ablation procedures targeting different anatomical locations.
STENTYS’ next-generation of self-apposing stent system receives CE mark ahead of plan
STENTYS will officially launch Xposition S at the EuroPCR conference on 19 May, 2015.
First and only intravenous antiplatelet agent receives the CE mark
The Medicines Company has announced that it has received the CE mark for cangrelor (Kangrexal) the first and only intravenous antiplatelet agent that provides immediate, consistent, and rapidly reversible P2Y12 inhibition.
First patient enrolled in Vessix sham-controlled study
Boston Scientific has announced that it is taking a new approach to evaluate the performance of its Vessix renal Denervation System. It says it is initiating a study with a novel design to isolate the effects of the therapy in patients with high blood pressure.
FDA approves feasibility study for CardiAQ Valve Technologies’ transcatheter mitral valve implantation system
CardiAQ Valve Technologies has received FDA investigational device exemption approval for a US early feasibility study of its second-generation transfemoral and transapical transcatheter mitral valve implantation system.
First patient enrolled in Tendyne’s transcatheter mitral valve implantation trial
Tendyne has announced that its bioprosthetic mitral valve was successfully implanted in the first patient in the USA as part of a multicentre global feasibility study that aims to provide early insights into the safety and performance of the device.
Leading cardiovascular societies release new guidance on use of heart pumps
An expert consensus statement released today by the Society for Cardiovascular Angiography and Interventions (SCAI), American College of Cardiology (ACC), Heart Failure Society of America (HFSA) and The Society of Thoracic Surgeons (STS) provides new guidance to help physicians match the right device with the right patient.
Publication suggests intraaortic balloon pumps as first line choice for high-risk percutaneous coronary intervention patients
Maquet Cardiovascular USA has announced publication of a study comparing the impact of percutaneous ventricular assist devices with intraaortic balloon pumps for high-risk patients undergoing percutaneous coronary intervention.
Direct Flow Medical to broaden SALUS pivotal trial
Direct Flow Medical has received investigational device exemption approval from the US FDA to broaden its SALUS trial, including the addition of high risk patients and randomisation against Medtronic’s CoreValve.
FDA grants expanded labelling claim to On-X Life Technologies
Patients with On-X aortic heart valves may be able to reduce their regular blood-thinning medication regimen, thanks to an expanded labelling claim granted by the US FDA to On-X Life Technologies.
PICSO system has potential to improve myocardial recovery after PCI
The first safety and feasibility study of pressure-controlled intermittent coronary sinus occlusion (PICSO) in the setting of ST-segment elevation myocardial infarction indicates that the system could potentially enhance myocardial recovery after primary percutaneous coronary intervention.
Prognosis is significantly worse with non-access site bleeding
A meta-analysis of site-specific bleeding after percutaneous coronary intervention indicates that non-access site bleeding is associated with a significantly worse prognosis than is access-site bleeding.
Robotic PCI could be used to reduce radiation exposure to the operator
Ryan Madder, an interventional cardiologist at the Frederik Meijer Heart & Vascular Institute (Spectrum Health, Grand Rapids, Michigan, USA) explains why he believes a robotic system (CorPath, Corindus Vascular Robotics) for performing percutaneous coronary intervention (PCI) may help to reduce radiation exposure.
ORSIF created to advocate for safety in hospital catheterisation labs
The Organization for Occupational Radiation Safety in Interventional Fluoroscopy is a non-profit association raising awareness of health risks associated with fluoroscopy in catheterisation labs and radiographic diagnostic laboratories.
Stem cells may slow the heart’s ageing process
A new study published in Stem Cells Translational Medicine demonstrates how mesenchymal stem cells not only protect the heart from further damage after a cardiac incident but can also slow down its ageing process.
FDA approves investigational study for new smaller SynCardia Total Artificial Heart
The FDA approval will allow SynCardia to launch the study with as many as 30 heart failure patients, who will receive the 50cc SynCardia Total Artificial Heart as a bridge to a donor heart transplant.
CardioCel to feature at prominent heart valve conferences
Admedus' CardioCel will be featured in a number of presentations at the American Association for Thoracic Surgery Mitral Conclave 2015 meeting and the 95th AATS Annual Meeting.
Biotronik announces European launch of Pantera Pro coronary balloon catheter
The workhorse balloon is designed to enhance surgical access to difficult lesions and challenging patient anatomies.
CoreValve is now approved for valve-in-valve procedures in the USA
The FDA has approved the use of CoreValve for aortic valve-in-valve replacement procedures, meaning the transcatheter aortic valve implantation (TAVI) device is the first such device to be approved for valve-in-valve procedures both for patients who are at high risk and for those who are extreme risk for surgery in the USA
Study demonstrates concern for retained blood syndrome after heart surgery
Cardiac anaesthesia investigators from Germany presented data at the International Anesthesia Research Society's (IARS) 2015 Annual Meeting and International Science Symposium.
Toshiba launches single-lane SPDT switches supporting 3rd generation PCI Express
The new TC7PCI3212MT and TC7PCI3215MT support PCI Express Gen3 (8Gbps) and achieve wide bandwidth characteristics of 11.5GHz at -3dB.
Sorin Group signs merger agreement with Cyberonics
The transaction remains subject to conditions including approval by both Sorin and Cyberonics' shareholders, the receipt of required antitrust and regulatory clearances, and other customary closing conditions.
First US commercial procedures with the Watchman device
Watchman offers a stroke risk reduction option for high-risk patients with non-valvular atrial fibrillation who are seeking an alternative to long-term warfarin therapy.
Surgery is still BEST for multivessel disease in the long run
The results of the BEST study indicate that, in patients with multivessel disease, coronary artery bypass grafting (CABG) is associated with significant better outcomes than percutaneous coronary intervention (PCI) even in the era of second-generation drug-eluting stents. However, a registry study has indicated that there is no difference in mortality rates between PCI with everolimus-eluting stents and CABG
SERVE-HF could be a wake-up call for cardiologists about central sleep apnoea
Martin Cowie (Imperial College London, London, UK) is the principal investigator of the SERVE-HF study, which is assessing the use of adaptive servo-ventilation (PaceWave, ResMed) in chronic heart failure patients with central sleep apnoea. In this interview he explains why he believes the study, if positive, could convince cardiologist to start screening for the condition.
CardioKinetix announces results of Chinese trial evaluating Parachute heart failure device
CardioKinetix has announced three-month clinical and echocardiographic results of PARACHUTE China, a study of 31 Chinese patients treated consecutively with the company's Parachute ventricular partitioning device.
First patient enrolled in all-comers randomised trial of MiStent compared with Xience
DESSOLVE III is a randomised, controlled clinical trial comparing the MiStent sirolimus eluting absorbable polymer coronary stent system with the Xience everolimus eluting coronary stent system.
Abiomed Impella 2.5 receives FDA approval for percutaneous coronary intervention
Impella 2.5 is a ventricular support device used during high risk percutaneous coronary intervention in elective or urgent haemodynamically stable patients with severe coronary artery disease and depressed left ventricular ejection fraction.
HeartIT launches CloudCMR
HeartIT has launched CloudCMR, designed to promote worldwide sharing of de-identified cardiovascular magnetic resonance images for education, research, and quality control purposes.
TriGuard increases proportion of TAVI patients free from ischaemic brain lesions
The DEFLECT III trial indicates that use of the cerebral protection device TriGuard (Keystone Heart) in patients undergoing transcatheter aortic valve implantation (TAVI) is associated with an increase in the proportion of patients free from ischaemic brain lesions.
Favourable MitraClip trial data from initial presented at ACC 2015
MitraClip is a treatment option for degenerative mitral regurgitation patients who are not good candidates for surgery the current standard of care because of their advanced age, frailty or other complicating factors.
Toshiba’s Infinix 4DCT receives FDA clearance with Aquilion PRIME CT configuration
Toshiba showcased the Infinix 4DCT with the Aquilion PRIME CT at the American College of Cardiology annual meeting (14–16 March, San Diego, USA).
Boston Scientific receives FDA approval for Watchman left atrial appendage closure device
The device offers an alternative to long-term warfarin therapy for stroke risk reduction in patients with non-valvular atrial fibrillation.
CoreValve system sustains superior survival benefit over open heart surgery at two years
The researchers presenting the results at the American College of Cardiology 2015 meeting late-breaking clinical trial suggested that self-expanding TAVR should be considered as the new standard of care.
Medtronic to initiate clinical study of Drug-Filled Stent following successful preclinical results
New platform enables controlled, polymer-free drug elution and is designed to help address next-generation technology challenges.
Study shows Brilinta treatment reduced thrombotic cardiovascular events in patients with a history of heart attack
Data from 21,000 patient study was presented at American College of Cardiology 64th Annual Scientific Session and simultaneously published in the New England Journal of Medicine.
CHAMPION data show significant improvement in survival rates for heart failure patients with reduced ejection fraction
Patients treated with the CardioMEMS heart failure system had a 57% reduction in mortality and a 43% reduction in heart failure hospitalisations compared with guideline directed medical therapy.
Philips introduces IntelliSpace Cardiovascular at the American College of Cardiology Meeting
IntelliSpace Cardiovascular is a web-enabled image and information management system with an integrated workspace producing a holistic view of a patient’s care continuum across the entire cardiovascular service line.
Siemens offers solutions for sustainable cardiovascular care at ACC 2015
Siemens offered a portfolio of systems and support solutions ranging from imaging modalities to information technology to in vitro diagnostics,.
JACC reports on first-in-human transcatheter tricuspid repair
Mitralign transcatheter annuloplasty system could present a non-surgical option for both mitral and tricuspid valve regurgitation.
Ablative Solutions receives innovation award
Ablative Solutions was the recipient of a "Top Cardiovascular Innovation Award" from Cardiovascular Research Technologies for the Peregrine system.
Medtronic initiates pivotal studies of Resolute Onyx drug-eluting stent in USA
Drug-eluting stent device with CoreWire Technology is to be studied in a wide spectrum of patients, including the smallest coronary arteries.
Infraredx to launch TVC Imaging System and TVC Muller Extended Bandwidth NIRS-IVUS Catheter at ACC 2015
Research supporting the value of adding NIRS-IVUS imaging to intravascular cardiology practice for detecting coronary artery disease will be presented throughout the ACC meeting.
CREDIT II stent trial completes enrolment
CREDIT II is the first randomised controlled trial involving the Excel II coronary stent. Excel II is the latest generation in the Excel family of biodegradable polymer drug-eluting stents.
Cardiac Dimensions completes US$43m equity financing
US$15.2m investment from international investors rounded out financing. The funds will support a landmark trial and expand the company’s commercial operations.
Admedus expands Cardiocel market and anticipates record sales quarter
Admedus' CardioCel has entered the market in Hong Kong, continuing the expansion of CardioCel into Asian markets as part of Admedus' global product launch strategy.
Forge Medical reports clinical outcomes of VasoStat haemostasis device at ECR 2015
Initial clinical outcomes of Forge Medical's VasoStat haemostasis device were presented at the European Congress of Radiology (ECR) (4-8 March, Vienna, Austria).
Sunshine Heart pauses enrolment in US pivotal study of C-Pulse heart assist system
This pause in enrolment is in accordance with the study protocol where in the event that more than three of the first twenty subjects pass away for any reason, the company will work with the FDA to discuss a plan to resume enrolment.
Bracco Imaging launches the EmpowerCTA+
The EmpowerCTA+ has initially been launched in the USA and gradually in Europe, and was presented at the upcoming European Congress of Radiology (4–8 March, Vienna, Austria).
Drug-coated balloons are not inferior to drug-eluting stents for treating stent restenosis
A retrospective study indicates that drug-coated balloons are associated with similar angiographic and clinical outcomes to drug-eluting stents (including both first- and second-generation stents) for the management of in-stent restenosis.
Peritoneal hypothermia is feasible but does not reduce infarct size in STEMI patients
A new study indicates that while peritoneal lavage is a feasible method of achieving rapid cooling in patients with ST-segment elevation myocardial infarction (STEMI), it does not reduce infarct size and is associated with a significantly increased rate of adverse events.
OrbusNeich appoints Alain Aimonetti as vice president
Prior to joining OrbusNeich, Aimonetti held a variety of senior roles in the cardio- and endovascular medical devices industry.
Flavio Ribichini
Flavio Ribichini speaks to Cardiovascular News about being involved in the first use of primary angioplasty in Italy and how his belief in the "learning-teaching continuum" informs his clinical practice.
PleuraFlow exhibited at Association of Physician Assistants in Cardiovascular Surgery Meeting
The PleuraFlow System uses a first-of-its-kind technology to enable caregivers to proactively keep chest drainage tubes clear of blood clotting after heart surgery.
Alvimedica launches Cre8 46mm
Alvimedica has announced CE mark in Europe for the Cre8 46mm polymer-free Amphilimus eluting stent system, dedicated to long blockages in the vessels due to coronary artery disease.
Aortic regurgitation measurement based on X-ray imaging receives 510(k) clearance
The quantitative regurgitation workflow provides quantification of aortic regurgitation by using density of contrast in the aortic root and ventricle based on X-ray aortogram images.
Cardiovascular Systems releases two-year ORBIT II coronary data
ORBIT II is a study of the company's Diamondback 360 coronary orbital atherectomy system (OAS) in treating severely calcified lesions.
Worse outcomes after TAVI for patients with moderate-to-severe kidney disease
A new study indicates that patients undergoing transcatheter aortic valve implantation (TAVI) who have moderate-to-severe chronic kidney disease have significantly worse outcomes than those undergoing TAVI who have mild disease.
Meta-analysis indicates that bivalirudin does increase risk of early stent thrombosis
A new meta-analysis shows that bivalirudin significantly increases the risk of early stent thrombosis in patients undergoing percutaneous coronary intervention compared with other antithrombotic therapies. However, this increase in stent thrombosis is not associated with an increased risk of death or myocardial infarction.
Biotronik advances clinical evaluation of latest generation DREAMS scaffold
DREAMS is an absorbable scaffold that combines the mechanical advantages of a metallic stent with a reliable bioabsorption profile that keeps the vessels open while avoiding the long-term disadvantages of permanent metal stents.
CeloNova announces accelerated IDE trial enrolment completion for its Cobra PzF coronary stent
Completed enrolment was ahead of schedule and supports submission for FDA approval of the novel Cobra PzF stent with its advanced, nano-thin coating of Polyzene-F polymer.
SynCardia Total Artificial Heart patients experienced acceptable outcomes after one year
Seventy-four per cent of patients who were supported by the SynCardia temporary Total Artificial Heart for more than a year were either bridged to a donor heart or awaiting one.
Physician safety and stent savings with robotic-assisted PCI to be presented at CRT 2015
Paul T Campbell has explored the stent savings made possible when robotic percutaneous coronary intervention (PCI) is leveraged for the measurement of anatomy in place of manual methods of measurement.
Exploring new transcatheter options for the mitral valve
Anson Cheung (University of British Columbia, St. Paul's Hospital, Vancouver, Canada) speaks to Cardiovascular News about the potential benefits of transcatheter mitral valve implantation.
Medtronic announces CE mark and European launch of the Euphora semicompliant coronary balloon catheter
The first patient case with the Euphora was recently performed by Richard Edwards, consultant cardiologist at the Freeman Hospital in Newcastle, UK.
Asahi Intecc and Svelte Medical Systems announce manufacturing and co-branding agreements
Collaboration features latest Asahi technology in design, development and supply of core wire and coil assembly for the Svelte drug-eluting coronary stent fixed wire integrated delivery system.
Ablative Solutions welcomes hypertension leader to its Scientific Advisory Board
Michael Weber is currently a professor of medicine at SUNY Downstate College of Medicine in, New York City, USA and the editor-in-chief of The Journal of Clinical Hypertension.
St Jude Medical launches US study of paediatric mechanical heart valve
The HALO Trial represents a new opportunity for surgeons seeking a valve replacement for paediatric patients with no alternative approved treatment options.
Imaging heart muscle structure could give clues to heart disease
London researchers to develop new MRI technique to improve diagnosis.
Stentys announces the publication of new Self-Apposing stent in left main coronary artery study
Study showed superiority of Self-Apposing technology in opening the heart’s main artery.
LEADERS Free Japan trial completes enrolment
The LEADERS Free Japan is studying BioFreedom, the company's novel polymer and carrier-free drug-coated stent.
MicroPort receives CE mark approval for Firehawk
Approval allows MicroPort to commence commercialisation of Firehawk in the European market.
MiStent now commercially available in Europe
Novel drug-delivery technology provides sirolimus-eluting stent with unique bioabsorbable polymer absorption and drug-release profile.
First-of-their-kind imaging studies presented at TCT
The three studies were presented as late-breaking trials at the 2014 Transcatheter Cardiovascular Therapy (TCT) meeting (13–17 September, Washington, DC, USA).
Biotronik announces completion of patient enrolment for Orsiro BIOFLOW-IV study
The study is intended to support Japanese government approval of the Orsiro hybrid drug-eluting stent, which received CE mark in 2011.
Tailoring dual antiplatelet therapy duration for an individual patient
Guillaume Cayla explores how the duration of DAPT may be tailored after careful evaluation of both bleeding and ischaemic risk, taking into consideration the type of the device implanted.
Minimally invasive clip can repair mitral valve regurgitation
Recently approved by the Food and Drug Administration, the MitraClip holds together the mitral valve's leaflets to reduce the degree of regurgitation caused by mitral valve regurgitation.
Medtronic expands access to the CoreValve Evolut R System with new valve sizes
CE mark approval received for new 26mm and 29mm valves.
Bellerophon Therapeutics adds to drug and device development capabilities with management additions
Deborah A Quinn appointed vice president and medical lead for INOpulse programmes and Martin Dekker joins as vice president of device engineering.
Biosensors enters into distribution agreement with Veryan for BioMimics 3D stent
The agreement covers certain international markets, but excludes the USA and Japan.
Analysis shows first significant advantages for transcatheter aortic valve in patients with prior CABG
The analysis was presented at the 2015 Annual Meeting of The Society of Thoracic Surgeons in San Diego, USA.
JenaValve appoints Jan Keltjens as interim chief executive
He replaces David J Drachman, and will assume the role effective immediately and until a permanent replacement for Drachman has been hired.
Sorin announces enrolment completion for the Perceval IDE US study
The study is being conducted to support submission for FDA approval for the novel sutureless aortic valve.
NICE issues FAD recommendation of Xarelto to reduce risk of secondary events in acute coronary syndrome
The Final Appraisal Determination is the final phase in a multi-step review process by NICE and recognises the improved patient outcomes using Xarelto as a treatment option on top of dual antiplatelet therapy.
On-X Life Technologies to launch Chord-X mitral valve chordal repair system
The On-X LTI system for mitral valve chordal repair is produced with On-X LTI’s proprietary ePTFE suture and the company says it will provide surgeons new tools to help simplify the procedure.
Abbott’s troponin test may help doctors double the diagnosis of female myocardial infarction
Results showed that Abbott's test was able to diagnose a myocardial infarction in 22% of cases for women compared to a standard of 11%, when using a sex-specific threshold.
FEops closes a €1.3m series A financing round to commercialise its TAVIguide service
The funding will be used to support the launch of FEops' first product, TAVIguide a cloud-based pre-operative planning service for transcatheter aortic valve implantation in key markets in Europe and the USA.
Transapical TAVI can be performed with comparable results to transfemoral TAVI in high-volume centres
Results from a registry of 1,000 patients undergoing transcatheter aortic valve implantation (TAVI) in a high-volume centre do not indicate a significant difference in the rates of short- and long-term mortality between patients undergoing transapical TAVI and those undergoing transfemoral TAVI.
Ultrasound has potential for detecting potential heart attacks and stroke before symptoms arise
A study of portable ultrasound carried out in the USA, Canada and India has revealed the potential of this technology for detecting plaques in peripheral arteries in both developed and developing country settings.
Novella Clinical and the Cardiovascular Research Foundation to collaborate on clinical trial expertise
The preferred provider collaboration will offer a set of clinical trial services to developers of cardiovascular drugs and devices.
ICI meeting is a “fruitful ground for new developments” in interventional cardiology
Rafael Bayer, co-chairman of the Innovation in Cardiovascular Intervention (ICI) meeting (14–16 December 2014, Tel Aviv, Israel), speaks to Cardiovascular News about the event's unique features.
Live from The Hammersmith: Fractional Flow Reserve in multi-vessel disease assessment
Justin E Davis, Imperial College, London, UK, presents a live case demonstration of the use of coronary physiology for the assessment of multi-vessel disease, using fractional flow reserve was to identify significant from non-significant coronary stenoses.
NuVascular Technologies and Worcester Polytechnic Institute to commercialise stem cell treatment for heart damage
BioGenerator, a minimally invasive nanotechnology solution, will treat the leading cause of US deaths.
Transcatheter Technologies to expand portfolio with Tresillo TMVI system
The pre-mounted, valved stent is folded without the need of an outer sheath, thereby enabling precision controlled positioning and also allowing for repositioning if needed.
Micro Interventional Devices completes STASIS clinical trial enrolment
The secure transapical access and closure study(STASIS) is a non-randomised, multicentre, CE mark study, evaluating the safety and performance of Permaseal in transcatheter valve replacement procedures.
HeartFlow names new general counsel to executive team
Noemi C Espinosa brings to HeartFlow 30 years of experience as both a business executive and outside counsel, representing medical device, life science and high tech companies.
CytoSorbents submits IDE application for US CytoSorb cardiac surgery trial
The goal of CytoSorb treatment is to reduce inflammatory mediators and proteins such as cytokines and plasma free haemoglobin generated during surgery that can lead to serious post-operative complications.
Transcatheter Technologies introduces transfemoral Trinity TAVI system
Transcatheter Technologies GmbH has expanded the Trinity technology platform to include a transfemoral version.
Societies publish data standards for cardiovascular endpoints in clinical trials
The American College of Cardiology and the American Heart Association have released clinical data standards for cardiovascular and endpoints in clinical trials.
First clinical trial implant of the Tendyne transcatheter mitral valve a success
The Tendyne transcatheter mitral valve implant was successfully placed in the first patient enrolled in a three-continent, multicentre trial being conducted as part of the Tendyne feasibility study.
CHART-1 phase III trial enrols 240th patient
Cardio3 BioSciences has announced the enrolment of the 240th patient in its CHART-1 European trial for C-Cure, the first and only stem cell therapeutic using guided stem cells for the treatment of congestive heart failure.
Sorin announces FDA clearance and first US implant of Memo 3D ReChord annuloplasty ring
The Memo 3D ReChord incorporates a chordal guide system into the existing Memo 3D ring to simplify and standardise the approach to artificial chord replacement.
First transcaval valve replacement procedure completed in Europe
Henry Ford cardiologist Adam Greenbaum was asked to share the technique with Markus Kasel at the German Heart Centre Munich.
CVRx granted humanitarian device exemption approval for Barostim neo legacy device
This decision represents CVRx's first commercial approval in the USA, based on an FDA determination that neo legacy is safe and can be used in US patients defined as responders to the Rheos Carotid Sinus Lead System.
Volcano announces 1,000th system activated with iFR modality worldwide
More than 1,000 systems have been activated with Volcano's instant wave-Free Ratio, allowing physicians and patients to benefit from a simplified workflow and a reduced need for hyperaemic agents.
HeartFlow appoints William C Weldon to board of directors
HeartFlow has named former Johnson & Johnson chairman and chief executive officer William C Weldon as a new member of its board of directors.
Heart charity offers novel technology grants
Heart Research UK is accepting outline applications for its latest Novel and Emerging Technologies (NET) grant round worth up to £250,000.
CVRx granted CE mark for the Barostim neo system for conditional MRI compatibility
CVRx has been granted CE marking to expand labelling of the Barostim neo system as safe for use in magnetic resonance imaging (MRI) systems under specified conditions.
Performing TAVI in a hybrid operating room
Daniel O' Hair believes hybrid ORs may provide distinct advantages for complex TAVI cases. He speaks to Cardiovascular News about his experience of performing TAVI in a hybrid OR.
Near-infrared spectroscopy may assist in predicting the risk of future cardiovascular events
Infraredx has announced results from an independent, prospective outcomes study evaluating the ability of intravascular near-infrared spectroscopy to identify lipid core-containing plaques.
Successful first human implants of the Tendyne Transcatheter Mitral Valve Implant
The Tendyne Transcatheter Mitral Valve system has been successfully implanted in three patients at the Royal Brompton Hospital in London, UK, under a compassionate use protocol.
GulfPCR-GIM 2014
The 4th edition of GulfPCR-GIM 2014, which takes place in Dubai, UAE, will bring together interventional cardiologists and cathlab staff from throughout the world to share experience and knowledge in order to improve cardiovascular care for all patients.
Too early to say that renal denervation is a failed therapy
In a new consensus statement, the Joint UK Societies claim that the negative results of SYMPLICITY HTN-3 should not be used as a "rationale for abandoning" renal denervation as a "novel therapeutic development" for resistant hypertension.
REVA implants first patients with Fantom scaffold
REVA Medical has initiated patient enrollment with its Fantom bioresorbable drug-eluting scaffold.
BIOTRONIK completes enrolment for BIOHELIX-I PRO-Kinetic Energy stent trial
BIOTRONIK has enrolled the final patient in the BIOHELIX-I clinical trial to evaluate the safety and efficacy of the PRO-Kinetic Energy coronary bare metal stent.
Neovasc announces treatment of first patient in TIARA-I clinical trial
Neovasc has enrolled the first patient in the European arm of its TIARA-I early feasibility trial, a multinational, multicentre trial being conducted at centres in the USA, Europe and Canada.
Philips launches DoseWise Portal radiation dose management software
Royal Philips has introduced the DoseWise Portal, a comprehensive radiation dose management software solution aimed at managing radiation exposure risk to patients and their caregivers.
Combined mitral valve repair and CABG do not provide additional benefits
The combination of coronary artery bypass grafting (CABG) and mitral valve repair is not associated with additional benefits compared with CABG alone in patients with multivessel disease and moderate functional mitral regurgitation.
First transcatheter tricuspid valve replacement performed in the USA
A team at Henry Ford Hospital, Detroit, USA, has successfully performed the first transcatheter tricuspid valve replacement surgery in the USA.
Baylis Medical expands European reach with new UK office
Baylis Medical has announced the official opening of its new office in Watford, UK, on the outskirts of London.
FDA allows marketing of non-invasive device to help evaluate heart blood flow
The US Food and Drug Administration has cleared the marketing of the HeartFlow FFR-CT software, which permits health care professionals to non-invasively evaluate blood flow in the coronary arteries of patients showing signs and symptoms of coronary artery disease.
Investors express confidence in Emblok embolic protection catheter with US$5m financing
Innovative Cardiovascular Solutions has completed a Class A Unit financing totalling US$5m to fund its Emblok embolic protection catheter.
SCAI recommends one year of dual antiplatelet therapy following drug-eluting stent placement in response to DAPT study
THE SCAI has responded to a study presented at the AHA Scientific Sessions examining the risks and benefits of continuing dual antiplatelet therapy beyond one year after placement of one or more drug-eluting stents as compared with aspirin therapy alone.
Toshiba Dose Tracking System awarded Novation’s Innovative Technology Designation
Toshiba America Medical Systems' Dose Tracking System (DTS) was awarded the Innovative Technology designation by Novation at its Innovative Technology Expo.
Surgeon-designed tool could be used to prevent acute and subacute complications after cardiac surgery
Complete evacuation of blood from the pleural and pericardial spaces after cardiac surgery is critical. Clearflow explain that retained blood in these areas can cause acute and subacute complications (such as tamponade).
Qvanteq AG enrols first patient in QUEST I clinical study
Qvanteq AG has enrolled the first patient in the First in Man clinical study QUEST I, following earlier regulatory approval from the Dutch and Swiss authorities.
BioCardia CardiAMP phase III clinical trial protocol receives FDA clearance
BioCardia has received permission to begin a phase III clinical trial of its bone marrow-derived CardiAMP therapy for heart failure after clearance from the US Food and Drug Administration (FDA).
New data from EVOLVE clinical programme demonstrate SYNERGY success
In the first successful US pivotal trial of a bioabsorbable polymer stent, the Boston Scientific everolimus-eluting bioabsorbable polymer platinum chromium coronary stent system met its primary endpoint.
Shockwave Medical announces late breaking results of Lithoplasty study
Shockwave Medical announced positive clinical results from the DISRUPT PAD study evaluating the safety and utility of Lithoplasty balloon catheters for the treatment of peripheral artery disease, at the Vascular Interventional Advances (VIVA) Annual Conference in Las Vegas, USA.
Injectable bioresorbable scaffold could revolutionise treatment after STEMI
A first-in-man study suggests that an injectable bioresorbable scaffold that is designed to prevent left ventricular remodelling after a large myocardial infarction is well tolerated and, therefore, could be a potential new therapeutic option for STEMI patients.
The data for thrombectomy are conflicting
Sanjit Jolly writes that data suggest that impaired microvascular perfusion after primary percutaneous coronary intervention (PCI) is strongly associated with subsequent mortality.1 Manual thrombectomy aims to reduce distal embolisation and improve microvascular perfusion during primary PCI.
Successful use of SynCardia temporary Total Artificial Heart
A 19-year-old woman was successfully implanted with the SynCardia temporary Total Artificial Heart and bridged to a donor heart transplant after cardiac surgeons used 3D virtual implantation to determine she was fit-eligible for the procedure, according to a case series in November's The Journal of Heart and Lung Transplantation.
UK “lags persistently behind” the rest of Europe in its management of structural heart disease
A multidisciplinary group of cardiac surgeons and interventional cardiologists, Heart Valve Voice, are calling for improved care of patients with structural heart disease in the UK, claiming that the country is "lagging behind" the rest of Europe in the number of aortic valve replacements and TAVI procedures it performs.
Essential Medical receives CE mark approval for X-Seal
Essential Medical has received CE mark approval for its new vascular closure device, X-Seal. X-Seal closes femoral arterial punctures made during cardiac catheterisation procedures such as angiograms, angioplasty, and stenting.
Medtronic launches Resolute Onyx drug-eluting stent following CE mark approval
Medtronic has undertaken the international launch of its Resolute Onyx drug-eluting stent following the receipt of CE mark approval. The first live patient implant of the Resolute Onyx occurred during the XII International Course of Endovascular and Myocardial Therapy in Madrid, Spain.
STENTYS secures agreement with Micell to distribute its novel drug-eluting stent
STENTYS has entered into a five-year agreement with Micell Technologies to be the exclusive distributor of the MiStent coronary stent worldwide (excluding the United States, Canada, China, South Korea and Japan).
Stentys sirolimus-eluting stent receives CE marking
Stentys has announced it received CE marking for its sirolimus-eluting stent (SES). The CE marking will allow the company to market its SES in Europe immediately and, starting in 2015, in the other countries where the company has commercial activity.
Medtronic begins clinical study of investigational pericardial surgical aortic heart valve
Medtronic has initiated the PERIGON (pericardial surgical aortic valve replacement) pivotal trial, a global, prospective clinical trial evaluating an investigational surgical aortic heart valve made from bovine pericardial tissue that is intended to replace a diseased, damaged or malfunctioning native or prosthetic aortic valve.
ESC Congress to return to Barcelona and Munich
The ESC has now announced that the world's largest cardiology meeting will be held in Barcelona in 2017 and Munich in 2018.
CE mark approval and first implant of MEMO 3D ReChord
The first implant was presented at the 28th Annual Meeting of the European Association for Cardiothoracic Surgery (EACTS) by Mattia Glauber.
NICE issues interim ACD recommending Xarelto (rivaroxaban) 2.5mg as an option for secondary prevention in ACS
The National Institute for Health and Care Excellence (NICE) issued its appraisal consultation document (ACD) recommending Xarelto (rivaroxaban) 2.5mg twice daily as an option for secondary prevention in acute coronary syndromes.
Genetic predisposition to elevated LDL-C associated with narrowing of the aortic valve
In an analysis that included approximately 35,000 participants, genetic predisposition to elevated low-density lipoprotein cholesterol (LDL-C) was associated with aortic valve calcium and narrowing of the aortic valve.
TransMedics Organ Care System heart technology used to perform the world’s first series of adult human heart transplants from donors after circulatory death
The TransMedics Organ Care System (OCS) heart technology was used to perform the world’s first series of adult human heart transplants from donors after circulatory death (DCD donors) at St Vincent’s Hospital in Sydney, Australia.
TransMedics Organ Care System used to perform the world’s first series of adult human heart transplants from donors after circulatory death
The TransMedics Organ Care System (OCS) heart technology was used to perform the world's first series of adult human heart transplants from donors after circulatory death (DCD donors) at St Vincent's Hospital in Sydney, Australia.
Cardio3 Biosciences appoints Warren Sherman as chief medical officer
Cardio3 BioSciences, a company working on the discovery and development of regenerative, protective and reconstructive therapies, has announced the appointment of Warren Sherman as chief medical officer, effective as of 1 November 2014.
HealthTap launches first USA-wide mobile health marketplace
The new HealthTap Concierge enables US doctors to connect privately with their own patients via HD video or secure text, and conduct paid consults at their convenience.
Restenosis at routine control angiography increases risk of death at four years
The four-year mortality rate is significantly higher in patients with evidence of restenosis at routine control angiography than patients without evidence of restenosis. The rate is significantly increased even in patients with restenosis who are asymptomatic.
Sunil Rao
Sunil Rao speaks to Cardiovascular News about his career highlights, including his research into the transradial approach and exploring ways to improve the safety of antithrombotic drugs.
Three studies show benefits of Perceval sutureless valve
Three studies presented at the 2014 EACTS annual meeting showed positive data for Sorin's sutureless Perceval aortic valve.
First patient treated with chemical renal denervation in Peregrine Study
Ablative Solutions has announced that the first patient in the Peregrine Study has been treated with company's the investigational Peregrine system, which delivers agents directly to the peri-adventitial area of the renal artery.
CardioCel to be launched in Canada
Admedus has announced that it now has a medical device licence to market CardioCel in Canada, which means that Canada becomes the latest market in the global launch of the product following sales in Europe and the USA.
New study will evaluate use of a second-generation stent in under-served populations
Boston Scientific has initiated a new study of its second-generation everolimus-eluting coronary stent (Promus Premier) to evaluate its use in under-served patient populations, such as women and African Americans.
Merit Medical unveils new ThinkRadial website and educational initiative
Merit Medical has announced that it has launched a new website and educational initiative (thinkradial.com). The aim is to provide specialised training courses and information to help interventional cardiologists master the necessary skills.
Neovasc to initiate TIARA-I Trial after receiving conditional FDA approval
Neovasc has received FDA conditional approval to initiate its TIARA-I Trial in the USA. The
trial will evaluate the safety and performance of the company's Tiara mitral valve system.
New ultrasound contrast agent receives FDA approval
Bracco Diagnostics has receives FDA approval for a new ultrasound contrast agent Lumason (sulfur hexafluoride lipid-type A microspheres) for injectable suspension, which is indicated for adults with suboptimal echocardiograms.
Positive clinical trial results for PleuraFlow for the reduction of hospital complications after heart surgery
ClearFlow has revealed positive results from its PRO-ACT (Prevention of retained blood outcomes using active clearance technology) study, which is evaluating its PleuraFlow Active clearance technology system to prevent retained blood complications after heart surgery.
Direct aortic approach with CoreValve is safe and effective
Data from a late-breaking trial (ADVANCE Direct Aortic) presented at EACTS indicate that the direct aortic approach with the CoreValve TAVI system is safe and effective for aortic stenosis patients at who are not suitable for the transfemoral approach.
Interim data from PROACT study confirm patients with On-X Plus 1.5 valve may be safely maintained at anticoagulation levels below current recommended guidelines
Interim results from the PROACT (Prospective randomised On-X anticoagulation clinical trial) study indicate that patients with the On-X Plus 1.5 aortic heart valve may be safely managed at lower INR rates.
Impact of mental stress on heart varies between men and women
A new study of men and women who were already being treated for heart disease, published in the Journal of the American College of Cardiology, shows that men and women have different cardiovascular and psychological reactions to mental stress.
Ziehm Imaging wins Gold Stevie Winner at the International Business Awards
Ziehm Imaging has been awarded with the Gold Stevie Award in the category "New Product & Product Management – Health and Pharmaceuticals" in the 11th annual International Business Awards.
Orsiro drug-eluting stent launched in France
Biotronik has released Orsiro, a drug-euting stent that combines an active biolute coating and a passive probio coating, on to the French market. The release comes after a succession of studies demonstrating the safety and efficiency of the stent.
Admedus to showcase Cardiocel at EACTS
Admedus has announced its attendance at the European Association for Cardio-Thoracic Surgery (EACTS) 28th annual meeting (11–15 October, Milan, Italy) and will be showcasing CardioCel, its advanced cardiovascular scaffold, at the conference.
HeartPrint now listed as a Class 1 medical device
Materialise has listed its 3D-printed cardiovascular HeartPrint model as a medical device in the USA and EU markets. After years of 3D printing anatomical models for educational and research purposes, the company addressed the need for models that can assist with diagnosing, planning and practicing complex cardiovascular procedures.
First-in-man percutaneous repair of tricuspid valve performed
Mitralign has reported on the successful use of its technology to perform a percutaneous repair on a patient with tricuspid regurgitation. The company also announced that Joachim Schofer and Rebecca Hahn presented details of the procedure at PCR London Valves.
Direct Flow Medical receives CE mark for its enhanced transfemoral delivery system
Direct Flow Medical has announced that it has received the CE mark for an enhanced delivery system for its TAVI valve. It says that the new delivery system allows for easy access and excellent trackability through calcified and tortuous anatomies.
Zero-Gravity suspended radiation protection system approved for use in Europe
CFI Medical has announced CE mark approval for its Zero-Gravity suspended radiation protection system floor unit, paving the way for its widespread use internationally. Biotronik will continue to act as the exclusive distributor of zero-gravity outside North America.
AHA/ACC recommends ticagrelor over clopidogrel for NSTE-ACS
AstraZeneca has announced that the American Heart Association/American College of Cardiology, in their updated the guideline for the management of patients with non–ST-elevation acute coronary syndromes (NSTE-ACS), has given Class IIa recommendation for the use of ticagrelor (Brilinta) over clopidogrel in patients with NSTE-ACS undergoing early invasive or ischaemia-guided strategy or receiving a stent.
First patient treated in US pivotal trial of cerebral protection device
The first patient in the randomised-controlled SENTINEL trial, which is evaluating the role of Sentinel cerebral protection device to reduce the risk of patients in patients undergoing transcatheter aortic valve implantation, has been treated.
Barostim neo system can now be used for heart failure in Europe
CVRx has received CE mark approval of the Barostim neo system for the treatment of heart failure. The system is already CE-mark approved for the treatment of resistant hypertension.
Final NICE guidance recommends high-sensitive troponin tests to help evaluate heart attacks more quickly
The National Institute for Health and Care Excellence (NICE) has recommended Abbott's Architect Stat High Sensitive Troponin-I (hsTnl) test, among others, to help doctors quickly rule out heart attacks in NHS emergency departments in England and Wales.
New enhanced contrast-monitoring technology introduced at TCT
At the 2014 Transcatheter Cardiovascular Therapeutics (TCT) meeting, Osprey Medical introduced its enhanced contrast-monitoring technology. The new Avert Plus system incorporates a new smart syringe and LCD display system with the existing Avert system.
Solo Smart implanted for first time in US
Sorin Group has announced that the first US implant of Solo Smart was performed by David Heimansohn, St. Vincent's Heart Hospital, Indianapolis, USA.
No severe paravalvular leak with Acurate Neo TF
Data presented at PCR London Valves (28–30 September, London, UK) indicate that the Acurate Neo transfemoral TAVI system, which recently received the CE mark, is not associated with any incidences of severe paravalvular leak.
The incremental costs of TAVI with CoreValve in high-risk patients are acceptable
Data from the US CoreValve High Risk study show that the incremental costs of TAVI with the CoreValve device in patients at high risk for surgery, compared with surgical aortic valve replacement, replacement are acceptable from a US perspective.
Percutaneous closure of patent foramen ovale reduces migraine with aura days
The PRIMA study shows that percutaneous closure of patent foramen ovale reduces migraine with aura days compared with medical therapy but does not reduce total migraine days
First patient enrolled in US trial of Lotus valve system
Boston Scientific has initiated the REPRISE III clinical trial, which a US study to evaluate the safety and effectiveness of the Lotus valve system in patients with severe aortic stenosis.
Azeem Latib
Azeem Latib (senior interventional cardiologist, San Raffaele Hospital and EMO-GVM Centro Cuore Columbus, Università Vita Salute, Milan, Italy) started his career in South Africa, relying on the hard work of his mother, numerous weekend jobs, and a community bursary to support his studies, but moved to Italy after meeting his "life-changing" mentor Antonio Colombo.
Low rate of target vessel revascularisation with Cobra PzF coronary stent
CeloNova BioSciences has announced that positive first-in-man clinical trial results indicate that its Cobra PzF coronary stent system, which has a nano-thin coating of polyzene-F polymer, is a safe and effective routine treatment for real-world and complex patients with heart disease.
CE mark for transfemoral Acurate neo
Symetis has announced that it has received the CE mark for its Acurate neo transfemoral system, which means it now offers (like market leaders Medtronic and Edwards Lifesciences) both transapical and transfemoral options for TAVI delivery.
Sustained safety and performance with Lotus valve at one year
New data indicate that Boston Scientific's TAVI device, Lotus, is associated with sustained safety and performance at one year with no cases of moderate or severe paravalvular aortic regurgitation.
Carillon mitral contour system is associated with significant and sustained improvements in mitral regurgitation
Cardiac Dimensions has announced that new long-term outcomes data from the TITAN II clinical trial of its enhanced Carillon mitral contour system showed significant and sustained improvements in mitral regurgitation, functional improvement, quality of life and reverse cardiac remodelling.
Acist launches high-definition IVUS
Acist Medical Systems showcased the first-ever high-definition intravascular ultrasound (HDi) system during two live cases at the Transcatheter Cardiovascular Therapeutics meeting.
Further evidence to support healing benefits of the Combo stent
Data presented at the Transcatheter Cardiovascular Therapeutics (TCT) meeting (13–17 September, Washington, USA) provided further evidence to support the healing benefits of the Combo dual therapy stent, which is the only drug eluting stent with active EPC capture technology.
Low mortality rate with Self-Apposing stent at two years
According to results from the APPOSITION III study, which was presented at the Transcatheter Cardiovascular Therapeutics meeting (13–17 September, Washington, USA), the Stentys' Self-Apposing stent is associated with a low rate of mortality at two years after a myocardial infarction.
Positive three-year results for the Mistent SES
Micell Technologies has announced that positive three-year clinical results from the DESSOLVE I and DESSOLVE II trials of its Mistent sirolimus-eluting stent with a biodegradable polymer were presented at the Transcatheter Cardiovascular Therapeutics (TCT) meeting (13–17 September, Washington, USA)
All study endpoints met in DIRECT II
Svelte Medical Systems reports that all endpoints in its DIRECT II study, which evaluated the company's drug-eluting coronary stent integrated delivery system, have been met. The study confirms results seen in prior studies in which the integrated delivery system demonstrated procedural time and cost savings.
One-year clinical data from European trial shows consistent, compelling positive results for patients treated with minimally invasive device for heart failure
CardioKinetix has released results of a pooled analysis study of the first-of-its-kind catheter-based Parachute ventricular partitioning device.
Significant patient benefits with Sapien valve demonstrated in five-year PARTNER trial data
Five-year clinical outcomes for inoperable patients treated in the PARTNER trial were presented as part of the late-breaking clinical trials session at the Transcatheter Cardiovascular Therapeutics (TCT) conference.
ABSORB II study shows Absorb is comparable to Xience drug-eluting stent
At one year, overall clinical outcomes for Absorb (Abbott) were comparable to Xience (Abbott), and people treated with Absorb experienced a significantly lower rate of angina.
FDA clearance for Medtronic NC Euphora coronary balloon
Medtronic has announced the US Food and Drug Administration (FDA) 510(k) clearance and launch of the NC Euphora noncompliant balloon dilatation catheter.
First US implants in clinical study of recapturable CoreValve Evolut R
Medtronic has announced the first US implants in the CoreValve Evolut R Clinical Study, which will evaluate the safety and effectiveness of the new Medtronic CoreValve Evolut R system.
Micro Interventional Devices initiates “first-in-man” clinical study of Permaseal
Micro Interventional Devices has reported the first successful clinical case using its Permaseal Cardiac Access and Closure technology. This new technology simplifies minimally invasive aortic valve replacement procedures.
New methods enhance the quality of myocardial perfusion imaging
In her PhD study, Tuija Kangasmaa, invented a method which makes it possible to reduce the imaging time by up to 50%, making the scan session easier for the patient.
CE mark approval for Enabler-C coronary catheter system
The CE mark of the Enabler-C coronary catheter system follows the successful first-in-man study of the Enabler-C that took place at the Institut Cardiovasculaire Paris-Sud.
Failure of TRO40303 may question whether reperfusion injury actually occurs in man
The MITOCARE study indicates that the novel agent TRO40303 does not provide any protective effect compared with placebo in preventing reperfusion injury in STEMI patients undergoing PCI. This study, combined with many failures in the field, has raised questions about whether or not reperfusion injury actually occurs in man.
CE mark for recapture CoreValve Evolut system
Medtronic has announced that it has received the CE mark for its 23mm CoreValve Evolut R system for transcatheter aortic valve implantation (TAVI). A company press release states that the novel, self-expanding valve and 14FR equivalent delivery system offers new capabilities that advance valve performance and deliverability during the procedure.
Corindus Vascular Robotics to host breakfast symposium at TCT 2014
Corindus Vascular Robotic will host the breakfast symposium "Robotic PCI: Precision and protection from occupational hazards" at the upcoming Transcatheter Cardiovascular Therapeutics (TCT; 13–17 September, Washington, USA).
Initial patient enrolled in BioFreedom USA
Biosensors has announced enrolment of the initial patient in BioFreedom USA, an investigational device exemption (IDE) feasibility trial designed to collect additional US-based safety and effectiveness data for BioFreedom, the company's novel polymer and carrier-free drug-coated stent.
Heart Hospital of Austin is first in Texas to implant new Portico re-sheathable TAVI device
Physicians at the Heart Hospital of Austin became the first in Texas to implant the new Portico re-sheathable transcatheter aortic valve implantation (TAVI) device as part of the PORTICO trial, which is a nationwide clinical study to examine the effectiveness of the new heart valve.
Self-Apposing stent has now been implanted more than 10,000 times
Stentys has announced that its Self-Apposing stent has been implanted more than 10,000 times in patients worldwide. A company press release reports that this milestone further illustrates the popularity of Stentys' technology among cardiologists in Europe and a growing number of regions globally.
Drug-coated balloons for the management of coronary artery disease
Robert Byrne writes that although drug-coated balloon therapy has been a significant innovation with relevance for everyday practice, convincing data in the coronary arena exists thus far only for the treatment of in-stent restenosis.
Pre-hospital ticagrelor does not improve coronary reperfusion before PCI in STEMI patients
Pre-hospital administration of ticagrelor does not improve coronary reperfusion before percutaneous coronary intervention patients with ST-segment elevation myocardial infarction. However, it may reduce the risk of stent thrombosis.
FDA 510(k) clearance for TAVI precision treatment planning application
Royal Philips has announced that it has received 510(k) clearance from the US Food and Drug Administration to market its precision planning application for transcatheter aortic-valve implantation (TAVI) treatments.
Claret Medical raises up to US$18 million in Series B financing to advance the field of cerebral protection
Proceeds will fund randomised, pivotal SENTINEL trial to study how cerebral protection may reduce the incidence of stroke in transcatheter aortic valve implantation (TAVI).
Do not use balloon angioplasty to treat drug-eluting stent restenosis
A network meta-analysis indicates that while drug-eluting balloons and drug-eluting stents are effective treatment options for drug-eluting stent restenosis, balloon angioplasty (by comparison) is not an effective treatment and should not be used.
Confounders can partly explain obesity paradox in STEMI patients
A new study indicates the obesity paradox, in which being obese seems to confer a mortality benefit, observed in some studies of patients with ST-segment elevation myocardial infarction (STEMI) can be at least partly explained by confounders.
Transitioning from transfemoral to transradial: Why the transradial approach is here to stay
William H Crowder believes that, despite some US interventional cardiologists' reluctance to use the approach, the transradial approach will become standard of care for coronary catheterisation procedures.
CE mark approval for Direct Flow Medical 23mm valve
Direct Flow Medical has received the CE mark for a 23mm sized valve as part of its transcatheter aortic valve system. The company has also received the CE mark for implantation of all of its valves without the use of contrast media, protecting patients from kidney injury during transcatheter aortic valve implantation.
Severity of sleep apnoea impacts risk of resistant high blood pressure
A new study shows a strong association between severe, untreated obstructive sleep apnoea and the risk of elevated blood pressure despite the use of high blood pressure medications.
SCAI statement on new report showing heart disease deaths dropped significantly in past decade
Efforts over the past decade to improve the quality of care for cardiovascular disease patients and increase the use of evidence-based treatments have led to a significant drop in the rate of hospitalisations and deaths, according to a new study in Circulation.
Boston Scientific and ASAHI INTECC announce joint development and manufacturing programmes
The two companies have reached an agreement to develop a new and differentiated FFR guidewire and next generation Boston Scientific Rotablator RotaWire.
B Wayne Johnson Named OrbusNeich president and chief executive officer
OrbusNeich has announced the appointment of B Wayne Johnson as president and chief executive officer, with responsibility for all commercial and clinical aspects of the company.
US Department of Justice closes investigation into PLATO clinical trial for Brilinta
AstraZeneca has announced that it has received confirmation from the United States Department of Justice that it is closing its investigation into PLATO, a clinical trial with Brilinta (ticagrelor).
First patients enrolled in study to evaluate dabigatran etexilate after PCI with stenting
The study will evaluate the efficacy and safety of the oral anticoagulant dabigatran etexilate in patients with non-valvular atrial fibrillation, who have undergone a percutaneous coronary intervention (PCI) with stent placement to widen their blocked coronary arteries.
SERVE-HF: largest trial assessing benefits of treating HF patients with sleep-disordered breathing
Next year SERVE-HF, the world's largest randomised trial ever conducted to assess the benefits of effectively treating heart failure patients with sleep-disordered breathing, will be reporting results - setting the scene for major changes in how cardiologists view and manage this prevalent co-morbidity.
Juventas Therapeutics completes enrolment of phase I/II RETRO-HF trial
Juventas Therapeutics has announced that it has successfully completed the phase I arm of the RETRO-HF clinical trial, and fully enrolled the phase II arm that is evaluating the safety and preliminary clinical efficacy for retrograde infusion of JVS-100 in patients with heart failure.
Journal review finds gene therapy for subset of heart disease patients “highly warranted”
Cardium Therapeutics has announced the publication of a review article in the Journal of Cardiovascular Pharmacology that concludes a gene therapy product promoting the growth of blood vessels is "highly warranted" to treat heart-disease patients who are either ineligible or poor candidates for traditional angioplasty, stent placement or bypass surgery.
MitraClip system gets US Medicare National Coverage Determination
The Centers for Medicare & Medicaid Services recently issued a National Coverage Determination that extends coverage for Medicare beneficiaries in the United States to Transcatheter Mitral Valve Repair with Abbott's MitraClip system.
JenaValve names Stefan Schreck chief technology officer
JenaValve Technology has announced that cardiovascular medical device industry veteran Stefan Schreck has been named the company's new chief technology officer, effective immediately.
Toshiba Introduces SURESubtraction Coronary at ESC 2014
Toshiba has announced that it will feature SURESubtraction Coronary at The European Society of Cardiology (ESC) congress (30 August–3 September, Barcelona, Spain).
Robotic PCI study reveals favourable results
The paper compared the outcomes of 40 patients that underwent PCI procedures at a single site in the PRECISE trial using the CorPath robotic system to the 80 consecutive patients who met the same inclusion criteria but underwent conventional, manual PCIs at the same centre.
The role of rotational atherectomy in contemporary PCI practice
James Cockburn, David Hildick-Smith, and Adam de Belder describe their recent study (published in the International Journal of Cardiology), which found that patients who underwent rotational atherectomy had a lower mid-term survival compared with patients who underwent conventional percutaneous coronary intervention (PCI).
HeartWare International appoints Katrin Leadley as chief medical officer
HeartWare International has announced the appointment of Katrin Leadley as chief medical officer effective 1 September, 2014.
Thoratec announces start of HeartMate PHP CE mark trial
Thoratec Corporation has announced that its CE mark clinical trial for HeartMate percutaneous heart pump (PHP) has commenced.
Study supports a minimalist approach to transfemoral TAVI
A study published ahead of print in the Journal of American Cardiology: Cardiovascular Interventions supports a minimalist transfemoral approach to transcatheter aortic valve implantation (TAVI) for the treatment of high-risk and inoperable patients with aortic stenosis.
FDA approves SynCardia Total Artificial Heart with SynHall valves
The OK gives the company control over the two critical components for heart manufacturing: The valves and the unique formula for segmented polyurethane solution, which is manufactured only by SynCardia.
A simple intervention could prevent acute kidney injury in one in five PCI patients
According to a new study, a simple cost-effective multicentre quality improvement intervention could prevent contrast-induced acute kidney injury in one in five patients undergoing non-emergent percutaneous coronary intervention.
Cytori ATHENA trials on clinical hold
The decision to place the trials on hold was based on a safety review of reported cerebrovascular events. Symptoms occurred in three patients, of which two patients' symptoms fully resolved within a short period of time and the third patient has had substantial resolution of symptoms.
James Thomas joins Northwestern Medicine’s Bluhm Cardiovascular Institute
An expert in cardiovascular imaging with a unique background in space medicine, Thomas will begin seeing patients at Northwestern Memorial Hospital in August. He is also a professor of medicine at Northwestern University Feinberg School of Medicine.
ACIST enters co-promotion agreement with Medtronic
ACIST Medical Systems has announced that it has entered into a strategic agreement with Medtronic to co-promote the world's first Rapid Exchange FFR (RXi) and High Definition IVUS (HDi) technologies in the United States.
Tryton Medical enrols first patient in the EXTENDED Access registry
Tryton Medical has announced that the first patient has been enrolled in the EXTENDED Access Registry (Tryton IDE XA registry), a single arm study of its Tryton Side Branch stent.
FDA approves Solo Smart aortic pericardial heart valve
Solo Smart from Sorin Group is the first aortic valve with a removable stent, providing native-like performance with the ease of implant of a stented valve.
CE mark approval for Crown PRT heart valve
Sorin Group has announced that it has been granted CE mark certification for its innovative stented aortic bioprosthesis Crown PRT (Phospholipid Reduction Treatment), now commercially available in Europe.
CE mark approval for Agent drug-coated balloon
According to Boston Scientific, the Agent drug-coated balloon provides physicians with an additional alternative to treat both in-stent restenosis and de novo small vessel coronary disease.
Boston Scientific launches Polaris imaging system
According to Boston Scientific, the Polaris imaging system offers enhanced ease-of-use and more powerful processing capabilities.
Results of first-in-human study of Trinity TAVI device reported in EuroIntervention
Transcatheter Technologies has announced that results of a first-in-human clinical study of its Trinity system have been published ahead of print publication in the July issue of EuroIntervention.
Cytori technology selected for NHLBI funded trial in LVAD patients
The Cardiovascular Cell Therapy Research Network has selected Cytori Therapeutics to supply adipose-derived regenerative cells for a clinical trial aimed at evaluating the safety and feasibility of treating patients with left ventricular assist devices.
NICE extends recommendations for prasugrel
Revised guidance from NICE, recommends once-daily, oral antiplatelet prasugrel (Efient), in combination with aspirin, as a cost-effective option for a wider group of acute coronary syndrome patients having primary or delayed PCI.
Miracor Medical begins European launch of next-generation PICSO impulse system
The new PICSO (Pressure-controlled intermittent coronary sinus occlusion) impulse system, consisting of the impulse console and impulse balloon catheters, is CE-marked and has already been used to treat patients in the UK, Ireland, and Hungary.
FDA clearance of CorMatrix ECM for vascular repair
CorMatrix Cardiovascular has announced that it has received US Food and Drug Administration (FDA) clearance to market the CorMatrix ECM (extracellular matrix) for vascular repair.
FDA approves Rebel platinum chromium coronary stent system
The Rebel stent offers the identical stent platform as the Promus Premier drug-eluting stent but without the everolimus drug.
Mitral regurgitation does negatively affect outcomes after TAVI
A meta-analysis supports previous findings that preoperative moderate-to-severe mitral regurgitation negatively affects outcomes after transcatheter aortic valve implantation (TAVI), but also indicated that there is a trend towards mitral regurgitation improvement after TAVI.
Use of delayed consent in HEAT-PPCI was “entirely ethical”
A senior researcher in biomedical ethics (David Shaw, Institute of Biomedical Ethics, University of Basel, Basel, Switzerland) has provided a robust defence of the controversial use of delayed consent in the HEAT-PPCI study, calling it "entirely ethical"
BIBA MedTech to launch Tavimonitor.org web application service
BIBA MedTech announces the release of the Tavimonitor.org website application for computer desktops and Windows Phone 8 devices.
InspireMD appoints James Barry as chief operating officer
InspireMD has announced the appointment of James Barry as chief operating officer. Barry, who has more than two decades of experience in the medical device industry, will be based in the company's Boston, USA headquarters.
CardioVascular institute trial provides treatment for left-sided heart failure
Through a percutaneous procedure now available through a clinical trial at PinnacleHealth CardioVascular Institute the first minimally invasive catheter-based device aims to restore normal geometry and function in the damaged left ventricle.
First patient enrolled in MASCOT registry
OrbusNeich has announced that the first patient has been enrolled in the Multinational abluminal sirolimus coated bio-engineered stent (MASCOT) post-marketing registry designed to assess the long-term safety and effectiveness of the Combo stent.
CE mark for new 25mm Lotus valve system
Boston Scientific Corporation has received CE mark approval and begun the European commercial launch of its new 25mm Lotus transcatheter aortic valve implantation (TAVI) system, complementing the currently available 23mm and 27mm valve sizes.
Paragonix files 510(k) pre-market notification with FDA for Sherpa Perfusion cardiac transport system
According to Paragonix, the Sherpa Perfusion cardiac transport system can be used for either hypothermic oxygenated perfusion or static storage of donor hearts during transport.
STENTYS expands marketing of the self-apposing stent in South America
STENTYS has established distributor agreements in Argentina, Chile and Colombia.
480 Biomedical awarded US$1 million phase II NHLBI contract to advance bioresorbable scaffold
The phase II funding from the National Heart, Lung, and Blood Institute (NHLBI) is to continue the development of a bioresorbable, self-expanding scaffold to treat paediatric pulmonary artery stenosis.
Clinical hold of REGULATE-PCI following voluntary halt of trial by Regado
The FDA has informed Regado that a clinical hold has been placed on all patient enrolment and dosing of either study drug in the ongoing phase 3 REGULATE-PCI trial.
Toshiba launches Infinix Essential, repositions Infinix product line
According to Toshiba, the Infinix Essential is an ideal value system that does not sacrifice performance. With its slim, off-centre C-arm design, the system allows steep angulations for optimised vessel profiling during cardiac interventions.
First patient enrolled in PROSPECT II study
Infraredx has announced the first patient enrolled in PROSPECT II, a multicentre, prospective study designed to assess the ability of intravascular imaging to identify non-flow obstructing vulnerable plaques.
JenaValve names David J Drachman chief executive officer
JenaValve Technology has announced that medical device industry veteran David J Drachman has been named the company's new chief executive officer, effective immediately.
CardiAQ obtains stay of further proceedings in Neovasc’s pending European patent application covering transcatheter mitral valve replacement
CardiAQ recently filed a Lawsuit against Neovasc in Germany alleging that Neovasc's pending European patent application is based upon CardiAQ technology that Neovasc obtained from CardiAQ in 2009 and 2010 during a confidential supplier relationship.
Thoratec acquires Apica Cardiovascular
Thoratec Corporation has announced that it has acquired Apica Cardiovascular Limited for an upfront cash payment of US$35 million and potential future clinical and sales milestones of up to US$40 million.
Transradial access predicts survival in NSTEMI patients
A new study suggests that transradial access in patients with non-ST-segment elevation myocardial infarction (NSTEMI) undergoing percutaneous coronary intervention is associated with reduced bleeding and reduced mortality compared with transfemoral access.
Incidental findings on imaging leads to worse outcomes for prospective TAVI patients
A study indicates that patients being assessed for possible transcatheter aortic valve implantation (TAVI) who have incidental findings on computed tomography aortogram are significantly less likely to receive TAVI as definitive therapy and a have worse overall come than patients without these findings.
New approach for aortic valve replacement is feasible
According to a report in The Journal of Thoracic and Cardiovascular Surgery, totally endoscopic aortic valve replacement (TEAVR) is technically feasible. Lead author of the report Marco Vola talks to Cardiovascular News about TEAVR and its potential role in the management of patients with aortic stenosis.
First European experience with ProTrack at St George’s Hospital London
The first cases using the ProTrack Pigtail wire in Europe were performed last month at St George's Hospital in London, UK. The ProTrack Pigtail wire is used during the transseptal procedure, a challenging minimally invasive technique used to cross the septum of the heart.
CorMatrix completes enrolment of patients in RESTORE
CorMatrix Cardiovascular has announced the successful enrolment and treatment of five patients in the first ever study evaluating the CorMatrix ECM delivered trans-epicardially for the improvement of myocardial function in patients at risk or suffering from congestive heart failure.
Spectranetics completes acquisition of AngioScore
The Spectranetics Corporation has announced that it has completed the acquisition of AngioScore for US$230 million in cash, along with additional contingent commercial and regulatory milestone payments.
Siemens workshop enhances diagnostic confidence and workflow efficiency within ultrasound
Siemens Healthcare recently hosted a series of echocardiography and workflow efficiency workshops across the UK. Using live demonstrations of the ACUSON SC2000, delegates were able to discover how continuous volume imaging can improve diagnostic confidence and workflow efficiency.
Philips and Salesforce.com announce a strategic alliance
Royal Philips and salesforce.com have announced a strategic alliance to deliver an open, cloud-based healthcare platform. The collaboration has already resulted in two clinical applications to be launched on the new platform later this summer: "Philips eCareCoordinator" and "Philips eCareCompanion."
Corindus Vascular Robotics receives North American New Product Innovation Award in interventional cardiology
Corindus's CorPath vascular robotic system is the first medical device to bring robotic precision and accuracy to coronary angioplasty procedures, which may improve clinical outcomes while increasing radiation protection for interventional cardiologists.
Fortis Hospitals performs coronary artery bypass surgery through the abdomen
Doctors at Fortis Hospitals, Bangalore, India, performed minimally-invasive trans-abdominal coronary artery bypass graft (CABG). The procedure was performed by making only a small incision in the upper abdomen, sparing the breast bone or the chest wall completely.
Simbionix transoesophageal echocardiography training simulator helps learners master clinical skills
The simulated procedure provides training in the evaluation of native valve diseases, prosthetic valve dysfunction, congenital or acquired heart diseases such as atrial fibrillations, coronary thromboses, coronary stenoses and more.
Novel anticoagulation system for PCI patients is effective at supressing ischaemic events without compromising bleeding
A novel anticoagulation system, comprising of a single-stranded RNA factor IXa inhibitor (pegnivacogin) and its reversal agent (anivamersen), was shown to be safe and feasible for supressing both ischaemic events and thrombotic complications with a favourable bleeding profile in patients with acute coronary syndromes undergoing percutaneous coronary intervention.
Faster full strut coverage with self-apposing stent
According to results presented at EuroPCR (20–23 May, Paris, France), a self-apposing sirolimus-eluting stent is associated significantly faster full strut coverage and better apposition at four months than a balloon-expandable zotarolimus-eluting stent.
LEADERS Free Japan trial enrols first patient
Biosensors International has announced enrolment of the first patient in LEADERS Free Japan, a trial involving BioFreedom, the company's novel polymer and carrier-free drug-coated stent.
HLT names Kevin Bassett general manager
HLT has announced the addition of Kevin Bassett as general manager. In his new role, Bassett will lead the development and achievement of HLT's strategic business objectives and milestones in collaboration with parent company, Bracco Group.
Sapien XT launching in USA
Edwards Lifesciences has announced that it has received US Food and Drug Administration (FDA) approval for its Sapien XT transcatheter aortic heart valve for the treatment of high-risk and inoperable patients suffering from severe symptomatic aortic stenosis.
British Cardiovascular Society response to open letter concerning NICE draft guidance on statins
Iain A Simpson, president of the British Cardiovascular Society, highlights that the letter sent to NICE regarding their draft lipid guidance, by a number of healthcare professionals, including the President of the Royal College of Physicians of London, does not represent the views of the cardiovascular healthcare professionals in the UK.
First patient enrolled in investigator initiated REDUCE study
The principal investigators of the REDUCE (Randomised evaluation of short-term dual antiplatelet therapy in patients with acute coronary syndrome treated with the Combo dual-therapy stent) trial have announced the enrolment of the first patient at Isala Hospital, Zwolle, the Netherlands.
CoreValve system receives FDA approval for high-risk patients
FDA approval is based on research that showed clinical outcomes at one year with the CoreValve system (Medtronic) were superior to open heart surgery, the current gold standard for aortic valve replacement.
Cardiovascular Systems announces coronary data at late-breaker SCAI 2014
One-year data from ORBIT II show strong rates of freedom from target revascularisation, cardiac death and major adverse cardiac events (MACE).
FDA clears of Chocolate PTCA balloon catheter
The US Food and Drug Administration (FDA) has cleared the Chocolate percutaneous transluminal coronary angioplasty balloon catheter (Chocolate PTCA), for balloon dilatation of the stenotic portion of coronary artery or bypass graft stenosis for the purpose of improving myocardial perfusion.
Manual thrombus aspiration associated with a higher rate of direct stenting
Data from the EXAMINATION trial indicates that manual thrombus aspiration prior to primary percutaneous coronary intervention (PCI) is associated with a higher rate of direct stenting and a lower rate of postdilation than no manual thrombus aspiration prior to primary PCI.
Sapien 3 heart valve could be used in intermediate risk patients
According to the 30-day outcomes of the Sapien 3 study, the Sapien 3 transcatheter heart valve is associated with a low mortality rate and a low stroke rate in transfemoral patients with aortic stenosis who are at high or intermediate surgical risk.
Stentys to acquire stent delivery technology for its next-generation of self-apposing stents
Stentys has announced the signing of the acquisition agreement of Cappella Peel Away Inc. (Delaware, USA) and its assets relating to a novel stent delivery system. The catheter technology will enable the implantation of the self-apposing stent in the same manner as a conventional balloon-expandable stent.
New research shows tomato nutrient can help prevent heart disease
After decades of researching why people with Mediterranean diets suffer fewer heart attacks and strokes, clinical researchers have pinpointed how lycopene, found in the skin of tomatoes, could reduce risks.
RESPOND post market registry of Lotus valve system initiated
Boston Scientific has initiated the RESPOND post market registry to assess real world performance of the Lotus valve system. The RESPOND registry will collect data on clinical outcomes and device performance in 1,000 patients implanted at 50 centres around the world.
“Interventional cardiology is losing more than 50% of the talent pool”
The Women Committee of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) has been set up to attain gender equality in interventional cardiology at the professional and patient level. The chair of the committee, Julinda Mehilli speaks to Cardiovascular News about why the comparative lack of women in interventional cardiology means that the specialty is "losing more than 50% of the talent pool".
David J Cohen
David J Cohen, director, Cardiovascular Research, Saint Luke's Mid America Heart Institute, Kansas City, USA, has conducted extensive research into the cost-effectiveness of interventional cardiology devices. He talks to Cardiovascular News about his career and why understanding the cost-effectiveness of devices is essential for the future of interventional cardiology.
OrbusNeich releases long-term data for world’s first and only dual therapy stent
Combo Dual Therapy stent has been shown to actively promote rapid endothelialisation and deliver long-term, true vessel healing.
Medtronic announces CE mark approval and launch of NC Euphora coronary balloon
Medtronic has announced CE mark approval and launch of the NC Euphora noncompliant balloon dilatation catheter. The NC Euphora balloon catheter is now available in Europe and other countries outside of the United States that recognise the CE mark. It is not yet available in the United States.
Enrolment now complete in LEADERS FREE study
With enrolment now complete, the baseline patient population data from Biosensors' LEADERS FREE study was presented for the first time at EuroPCR 2014 (20–23 May; Paris, France) by principal investigator Philip Urban.
Biosensors announces appointment of new CEO
Biosensors International Group has announced that Jose ("Pepe") Calle Gordo has been appointed the company's new chief executive officer, with effect from 1 November 2014.
EnligHTN III primary results show renal denervation is safe and effective
St Jude Medical has announced preliminary results from the EnligHTN III study, which found the company's second-generation EnligHTN renal denervation system provided safe and effective therapy for patients with drug-resistant, uncontrolled hypertension six months post-procedure.
Boston Scientific reports strong performance data for Synergy stent system
Boston Scientific reports positive three-year follow-up data for the EVOLVE clinical trial, comparing the safety and performance of the Synergy everolimus-eluting bioabsorbable polymer platinum chromium coronary stent system to the Promus element stent system.
Lotus valve system demonstrates strong performance and safety profile at six months
According to the results of REPRISE II, only 1.1% of patients treated with the Lotus TAVI device (Boston Scientific) experienced moderate paravalvular aortic regurgitation with no severe cases of aortic regurgitation reported.
Small vessel BIOFLOW-II subgroup analyses demonstrate top performance of Orsiro
Biotronik announced new results from the subgroup analyses of the BIOFLOW-II clinical trial at the EuroPCR 2014 congress in Paris, France. The results were presented by investigator Manel Sabaté, Hospital ClÃnico y Provincial de Barcelona, Barcelona, Spain.
SynCardia total artificial heart with SynHall valves receives CE mark approval
The CE mark gives SynCardia systems control over the last critical component for SynCardia heart manufacturing.
InspireMD to showcase embolic systems with MicroNet Technology and results from MASTER I trial at EuroPCR conference
InspireMD to present at European symposium titled: 'MGuard Embolic Protection Stent: The Importance of Thrombus Management in STEMI Primary PCI'
Freedom Solo demonstrates low mortality rate at one year
The results of its trial on the Freedom Solo (Sorin) for approval on the US market were presented at the 94th Annual Meeting of American Association for Thoracic Surgery (AATS) in Toronto, Canada.
A less invasive approach to cardiac assist device therapy
Holger Hotz (Charite University Medicine Berlin, Germany) writes about a novel, less invasive therapeutic option that has been developed to stop the progression of heart failure and to give patients the chance of recovery.
CE mark approval for DESolve 100 novolimus-eluting coronary scaffold system
The world's first scaffold with a dramatically thinner strut profile of 100µm (one hundred microns) is designed to degrade within one year, returning the patients' coronary vessel ultimately to its normal de novo state while providing market-leading deliverability and conformability.
St Jude Medical announces first implants in US study of the Portico transcatheter aortic heart valve system
St Jude Medical has announced the first patient implants of the Portico re-sheathable transcatheter aortic valve system occurred in US IDE trial (PORTICO trial).
IDE approval for BioFreedom US trial
Biosensors has announced that the US Food and Drug Administration (FDA) recently granted conditional investigational device exemption (IDE) approval for a US-based clinical trial of the BioFreedom polymer-free drug-coated stent system.
FDA clears GE’s new Discovery IGS 740 mobile angiography system
GE Healthcare has announced that it has received US Food and Drug Administration (FDA) clearance for its Discovery IGS 740, a new rail-free mobile angiography system with a 41x41cm detector. The Discovery IGS 740 is the first of this kind of mobile angiography system in the industry to receive FDA approval.
Ablative Solutions strengthens its Scientific Advisory Board
Ablative Solutions has announced that it has added Paul Sobotka, a highly respected clinician, to its Scientific Advisory Board.
UK babies set to receive heart test at birth
The UK's leading children's heart charity is delighted that all babies in the UK are set to receive a life-saving test to detect heart conditions at birth.
Leading cardiac surgeons share their experiences with new technology
Distinguished physicians from leading cardiac surgery centres gathered at an educational symposium to share their experiences with a new-generation blood evacuation system for use following cardiothoracic surgical procedures.
Positive results for iMOS Prime registry for the MGuard Prime embolic protection system
InspireMD has announced new results from the iMOS (International MGuard Observational Study) Prime registry demonstrating that use of the MGuard Prime in cases of acute ST-segment elevation myocardial infarction (STEMI) patients undergoing percutaneous coronary intervention resulted in complete ST-resolution in approximately 75% of cases, and a 2.2% rate of major adverse cardiac events at 30 days, including zero cases of mortality.
Fortis launches India’s first comprehensive centre for treatment of end-stage heart failure and transplant
Fortis has set up a world-class comprehensive centre for heart failure and heart transplant, at the Fortis Malar Hospital, in Chennai, India. The speciality centre under the leadership of K R Balakrishnan, director Cardiac Sciences and Suresh Rao K G, chief of Cardiac Anaesthesia and Critical Care, is ably supported by an experienced team of leading cardiac experts.
Eurocor launches Apertus aspiration catheter
The catheter is specifically designed for enhanced fast and accurate thrombus aspiration. The new device is being marketed in over 50 countries worldwide.
Medtronic reports 3f Enable five-year follow-up data
Medtronic has announced five-year follow-up data demonstrating the safety and performance of the 3f Enable aortic bioprosthesis - the world's first commercially available sutureless tissue heart valve.
Stentys’ self-apposing stent featured in Journal of the American College of Cardiology
Stentys has announced that the Journal of the American College of Cardiology (JACC) published a "State-of-the-Art Paper" entitled "Mechanisms, Pathophysiology, and Clinical Aspects of Incomplete Stent Apposition," which highlights Stentys' self-apposing stent among novel devices to minimise stent malapposition in clinical practice.
Positive outcomes for CoreValve patients treated via alternative access routes
Medtronic has revealed new data demonstrating positive outcomes for extreme risk patients with severe aortic stenosis who were treated with the CoreValve system via alternative access approaches.
Promus Premier everolimus-eluting coronary stent system launches in Japan
Boston Scientific Corporation has launched the Promus Premier everolimus-eluting platinum chromium coronary stent system in Japan. This advanced drug-eluting system recently received Japan Ministry of Health, Labour and Welfare approval.
FDA approves Mitroflow aortic heart valve with phospholipid reduction treatment
Sorin Group has announced that it received US Food and Drug Administration (FDA) approval for the Mitroflow aortic pericardial heart valve with phospholipid reduction treatment.
CE mark for TIVUS device
Cardiosonic has announced CE marking of its TIVUS (therapeutic intravascular ultrasound) ablative catheter device.
Volcano announces limited market release of SyncVision
SyncVision is currently installed in multiple limited market release sites throughout USA and Europe. According to Volcano, the new system allows co-registration of angiography with intravascular ultrasound imaging.
Cardinal Health to acquire AccessClosure
Cardinal Health has announced its entrance to the interventional cardiology arena with the purchase of AccessClosure for US$320 million.
Ochsner Medical Center buys Toshiba cardiovascular X-ray technology
Ochsner Medical Center has installed a new hybrid operating suite and cardiovascular X-ray system to conduct transcatheter aortic valve replacement and other coronary and peripheral procedures.
Bivalirudin use during percutaneous coronary intervention for non-ST segment-elevation acute coronary syndromes is associated with lower bleeding
A study has found that bivalirudin use during percutaneous coronary intervention (PCI) is associated with less composite bleeding when compared with unfractionated heparin monotherapy, in patients with non-ST segment-elevation acute coronary syndromes or stable ischaemic heart disease.
Peri-PCI bleeding in patients with diabetes carries “significant” increased risk of mortality
A study among patients with diabetes, drawn from seven randomised trials, has shown that bleeding within 30 days of percutaneous coronary intervention is associated with an increased risk of one-year mortality, non-fatal myocardial infarction or stent thrombosis.
Federal Appeals Court agrees to Medtronic request to postpone injunction
The Federal Circuit Court of Appeals has granted a request from Medtronic to postpone the implementation of an injunction that would have prevented the company from selling its CoreValve system in the United States.
STENTYS appoints medical device executive to its Board of Directors
STENTYS announced that Dianne Blanco has been appointed as a director to replace the representative from Omnes Capital venture capital fund.
Abbott completes enrolment of randomised clinical trials for Absorb
Abbott has announced that it has completed enrolment of three clinical trials to support approvals of its Absorb bioresorbable vascular scaffold in the United States, Japan and China. The product, which dissolves over time, will be compared with drug-eluting stents.
One-Year clinical data show positive results for patients treated with minimally invasive structural heart device
CardioKinetix announced results of a pooled analysis study of the first-of-its-kind catheter-based Parachute ventricular partitioning device. Twelve-month clinical results from 111 consecutive US and European patients with ischaemic heart failure were presented at the 2014 American College of Cardiology (ACC) Conference in Washington, DC, USA, by Philip Adamson, medical director at the Heart Failure Institute at Oklahoma Heart Hospital.
Lotus valve system demonstrates strong performance
The Boston Scientific Lotus valve system advanced transcatheter aortic valve implantation (TAVI) technology continued to demonstrate impressive performance at three months, according to new data presented at the American College of Cardiology conference 2014 in Washington, DC, USA.
JenaValve expands Series C venture financing round
JenaValve Technology announced that it has secured an expansion to its Series C venture round of US$10 million, increasing the total amount raised in the round from US$62.5 million to US$72.5 million.
CE mark approval for Intuity Elite valve system
Edwards Lifesciences announced it has received CE mark approval for the advanced Edwards Intuity Elite valve system. This next-generation rapid deployment system facilitates smaller incisions in surgical aortic valve implantation (AVI) procedures, and is built upon extensive evidence supporting the durability of the Carpentier-Edwards Perimount heart valve design.
CoveValve system demonstrates long-term durability
Medtronic announced the final follow-up results from the CoreValve CE pivotal study, which demonstrated excellent long-term durability at four years in patients with severe aortic stenosis who were treated with the self-expanding CoreValve system.
ACIST launches RXi rapid exchange fractional flow reserve system
ACIST Medical Systems announced the global introduction of the new ACIST RXi rapid exchange fractional flow reserve system - the world’s first rapid exchange fractional flow reserve system. This device features new technology designed to provide physicians with a fast and easy way to perform fractional flow reserve procedures.
CE mark for Tryton Medical left main indication
Tryton Medical announced that it has received CE mark approval for the treatment of left main coronary artery disease. With this approval, Tryton Medical becomes the first company to earn a CE mark for this indication.
CE mark approval for Perceval sutureless aortic valve extended age indication
Sorin Group announced Perceval, its sutureless aortic valve has received CE mark approval for adult age indication allowing treatment of a wider spectrum of patients with aortic stenosis and/or steno-insufficiency. Until now, only patients over 65 years of age could benefit from the Perceval technology.
American College of Cardiology elects Patrick O’Gara president
The American College of Cardiology (ACC) has elected Patrick O'Gara, as president for the year ahead at its 63rd Annual Scientific Session.
Data confirm the Neovasc reducer improves functional capabilities in refractory angina patients
Neovasc reported that final data from its COSIRA trial assessing the efficacy and safety of the Neovasc Reducer, a novel percutaneous device for the treatment of refractory angina, were presented on March 29, 2014 in a Featured Clinical Research Presentation at ACC.14, the American College of Cardiology 63rd Annual Scientific Session & Expo.
Boston Scientific platinum chromium coronary stent platform demonstrates low event rates through four years
Data from the PLATINUM Workhorse clinical trial demonstrate low event rates out to four years with Platinum chromium everolimus-eluting stent system confirming excellent long-term performance. At four years, the Platinum chromium everolimus-eluting stent system also continued to demonstrate advantages over the Cobalt chromium everolimus-eluting stent system.
Analysis of EUROMAX and HORIZONS-AMI trials of Angiomax (bivalirudin) presented at ACC
New pooled analysis of 5,800 patients shows bivalirudin is associated with reductions in cardiac death, major bleeding, transfusion and net adverse clinical events in STEMI patients undergoing percutaneous coronary intervention.
Melody transcatheter pulmonary valve shows positive clinical outcomes in real-world study
Medtronic announced the one-year results of the Melody transcatheter pulmonary valve US post-approval study, which found that real-world use of the Melody transcatheter pulmonary valve was associated with high procedural success, excellent valve function and few repeat procedures at the primary endpoint of six months.
OrbusNeich launches Sapphire II NC coronary dilatation catheter
OrbusNeich has announced the launch of the Sapphire II NC coronary dilatation catheter, a non-compliant balloon catheter engineered to cross tight lesions.
AccessClosure launches the next-generation Mynx Ace vascular closure device at ACC
AccessClosure commercially launched its Mynx Ace vascular closure device, a safe and secure vascular closure product that provides consistent results with a new, easy-to-use deployment system to seal femoral artery access sites.
CoreValve system results superior to open-heart surgery at one year in US pivotal trial
Medtronic announced that the CoreValve system showed results superior to surgical aortic valve replacement at one year in the High Risk Study of its CoreValve US pivotal trial, which evaluated patients with severe aortic stenosis who are considered high risk for surgery.
Medtronic releases results of SYMPLICITY HTN-3
Medtronic announced that the SYMPLICITY HTN-3 clinical trial, the first and only blinded, randomised, sham controlled study of renal denervation for treatment-resistant hypertension, met its primary safety endpoint but did not meet its primary or secondary efficacy endpoints.
Enrolment completed in European REMEDEE registry of Combo dual therapy stent
OrbusNeich announced the completion of enrolment in the prospective, multicentre, all-comers REMEDEE registry to evaluate the Combo stent for the treatment of coronary lesions in the setting of routine clinical care.
Takeda to present additional data from EXAMINE trial
Sub-analyses of the EXAMINE trial to be presented at the American College of Cardiology's 63rd Annual Scientific Session investigate the effects of alogliptin on cardiovascular mortality rates and hospitalisation for heart failure in type 2 diabetes patients with recent acute coronary syndrome.
New lifetime risk measure to predict heart disease
The key feature of the Joint British Societies Consensus Recommendations on the Prevention of Cardiovascular Disease is an innovative risk calculator: a web-based interactive tool, health professionals can use with patients to help communicate their longer term cardiovascular disease risk.
ORBIT II study one-year coronary data to be presented at American College of Cardiology Conference
Cardiovascular Systems will feature one-year data from the ORBIT II study of the company's Diamondback 360 coronary orbital atherectomy system.
Arterial Remodelling signs buyout agreement with Terumo
Terumo has obtained an exclusive option to purchase Arterial Remodelling Technologies' bioresorbable scaffold technology for use in the treatment of coronary artery disease.
Study identifies variables that predict MitraClip procedure failure
According to a new study, effective regurgitant orifice area, mitral valve orifice area, and transmitral pressure gradient are independent predictors of acute procedural failure in patients undergoing percutaneous mitral valve repair (MitraClip, Abbott Vascular).
Pre-hospital therapeutic hypothermia may not be beneficial
A recent study presented at the American Heart Association annual meeting (16–20 November, Dallas, USA) and published ahead of print in the Journal of the American Medical Association found that early therapeutic hypothermia (prior to hospital; immediately after return of spontaneous circulation) does not improve survival or neurological function in patients who have survived a cardiac arrest.
Feminine personality traits are a risk factor for poorer access to care
A study in the Canadian Medical Association Journal suggests that among younger patients with acute coronary syndrome, women have different access to care than men and feminine personality traits (in both men and women) are a risk factor for poorer access to care.
Transcatheter Technologies reports six-month follow-up results for pilot study of its Trinity TAVI system
According to the company report, at six-month follow-up, the mean pressure gradient was reduced from 59 mmHG at the start of the study to just 22 mmHG at six months post-implantation. Patients had zero AV-block or new pacemaker, and zero paravalvular leak.
FDA approves investigative device exemption trial for Cobra PzF
The IDE trial will study CeloNova's Cobra PzF, Polyzene-F stent technology in patients with heart disease. It will enrol patients in multiple research centres across the United States and in Europe.
On-X Life prepares to sell On-X Plus 1.5 aortic heart valve in Europe
On-X Life Technologies announced that it has assembled its European sales force at a training symposium in Barcelona ahead of launching its international marketing campaign for the On-X Plus 1.5 aortic heart valve.
FDA clears Volcano’s iFR Modality
Volcano Corporation has announced US Food and Drug Administration (FDA) clearance of its proprietary instant wave-free ratio (iFR) Modality and immediate commencement of US limited market release.
Neovasc Reducer featured in live case session at Congress of Update in Cardiology and Cardiovascular Surgery
The Neovasc Reducer for refractory angina was featured in a "live case" broadcast at the 10th Annual Congress of Update in Cardiology and Cardiovascular Surgery held in Antalya, Turkey.
Morph AccessPro steerable introducer gets CE mark approval
BioCardia announced receipt of the CE mark for its Morph AccessPro steerable introducer, designed for easier navigation through the vasculature during delivery of biotherapeutics and medical devices.
No extra benefit with dual antiplatelet therapy for aspirin-resistant CABG patients
The combination of aspirin and clopidogrel (or dual antiplatelet therapy) does not significantly reduce the risk of adverse events in aspirin-resistant patients who have undergone coronary artery bypass grafting (CABG) compared with aspirin monotherapy.
New smaller SynCardia Total Artificial Heart will expand therapy
Clinical trials awaiting US Food and Drug Administration (FDA) approval will document the use of a new smaller 50cc version of the SynCardia Total Artificial Heart, making it available to people of smaller size, including most women, men and many adolescents.
Neither valve design nor approach affect stroke rate after TAVI
A meta-analysis of transcatheter aortic valve implantation (TAVI) studies suggests that the design of a valve (eg. CoreValve vs. Sapien) does not affect the rate of stroke after TAVI. It also found that the approach (eg. transfemoral vs. transapical) also does not affect the incidence of stroke.
First human totally endoscopic aortic valve replacements (TEAVR) reported
Surgeons in France have successfully replaced the aortic valve in two patients without opening the chest during surgery. The procedure, using totally endoscopic aortic valve replacement (TEAVR), shows potential for improving quality of life of heart patients by offering significantly reduced chest trauma.
New partnership to launch remote robotics programme
The remote robotics programme envisions enabling remote robotic-assisted percutaneous coronary intervention for patients in rural areas. The remote robotics programme is the product of a partnership between Corindus Vascular Robotics and Sandford Health and the Leona M and Harry B Helmsley Charitable Trust.
Rebel platinum chromium coronary stent system gets CE mark approval
Boston Scientific Corporation has received CE mark approval for the Rebel platinum chromium coronary stent system, the company's latest generation bare metal stent for the treatment of coronary artery disease.
Coronary revascularisation in diabetic patients: Where do we stand?
Ehrin Armstrong reviews the revascularisation options and medical therapy for patients with diabetes and coronary artery disease.
Edwards Lifesciences announces first human implants with mitral transcatheter valve system
The first three human implants of the Fortis mitral transcatheter heart valve were performed in February and March by the Heart Team at St. Thomas' Hospital in London, UK.
Transcatheter Technologies wins ‘Best Business Pitch’ award at “German Venture Day” for its prosthetic aortic heart valve, Trinity
The Transcatheter Technologies Trinity TAVI system is designed to be the world's first 'truly repositionable' TAVI system.
Medtronic wins European Patent Office ruling
Medtronic announced that the European Patent Office has invalidated, in its entirety, the Edwards Lifesciences EP2055266 Spenser patent which was the basis for the August 26, 2013 injunction prohibiting sales of the CoreValve System in Germany.
Head-to-head independent study shows use of GE Healthcare’s Visipaque leads to better image quality
The study showed that use of isosmolar contrast agent Visipaque 320 (iodixanol 320mg I/ml) provides better image quality of coronary stents during multi-detector CT coronary angiography than use of Iomeron 400 (iomeprol 400mg l/ml) when injected at the same flow rate (5.0 ml/s) and volume (80ml).
Long-term safety and efficacy of MiStent SES presented at CRT 2014
Data presented showed rapid polymer absorption within three months coupled with drug delivery profile up to nine months, providing for excellent healing.
On-X Plus 1.5 aortic heart valve to launch at Society for Cardiothoracic Surgery in Great Britain & Ireland conference
On-X Life Technologies announced that it will launch its European marketing campaign for the On-X Plus 1.5 aortic heart valve in concert with its Great Britain distributor Vascutek at the Annual Meeting & Cardiothoracic Forum of the Society for Cardiothoracic Surgery in Great Britain & Ireland, March 10-12, Edinburgh, Scotland.
Philips drives digital imaging innovations at ECR 2014
Royal Philips will demonstrate advanced image quality, visualisation and clinical decision support at the European Congress of Radiology, March 6-10, in Vienna, Austria. Building on more than 20 years of digital imaging experience, the company is expanding its portfolio of integrated imaging solutions to improve access, optimise workflows and increase quality across the continuum of care.
Thoratec issues worldwide urgent Medical Device Correction Letter to update its labelling regarding the use of HeartMate II LVAS pocket system controller
Thoratec Corporation initiated a voluntary worldwide Medical Device Correction in order to update its labelling and training materials for the HeartMate II LVAS Pocket System Controller. This safety advisory is being issued because some patients and caregivers have experienced difficulties with the process of changing from a primary system controller to their backup system controller.
Toshiba partners with Unfors Raysafe for safer X-ray procedures
To improve clinical staff safety during interventional procedures and help monitor radiation dose, Toshiba Medical Systems Europe has partnered with Unfors RaySafe AB to offer a new dose monitoring and management tool for Infinix-i cardiovascular X-ray systems.
Enrolment complete in world’s first study of spontaneous tissue growth technology
Xeltis has announced that it has finished enrolment in a five-patient feasibility study of implantable products intended to enable for the first time the spontaneous growth of natural, healthy heart valves and vessels.
Guidelines provide new disease classification, lower threshold for intervention
New practice guidelines for the management of patients with valvular heart disease provide updated definitions of disease severity – categorising four progressive stages from "at risk" to "symptomatic severe" – and lower the threshold for intervention in select patient populations.
New imaging techniques to detect heart problems
New imaging techniques that can pinpoint the severity of a patient's heart disease are being researched by a team at King's College London thanks to a Novel and Emerging Technologies grant from national heart charity Heart Research UK.
Increased use of left ventricular assist devices could improve heart failure survival
Guy MacGowan reviews the benefits of using left ventricular assist devices to improve survival in patients with heart failure, and examines why there seems to be a reluctance to use the devices in the UK.
Fasting may not be necessary for PCI patients
The traditional practice of keeping patients nil-by-mouth for four-to-six hours prior to an elective percutaneous coronary intervention (PCI) procedure may not always be necessary as a retrospective study has found that not fasting before PCI is safe.
Baseline activated clotting time values could help to reduce bleeding after TAVI
In patients undergoing transfemoral transcatheter aortic valve implantation (TAVI), heparin administration guided by baseline activated clotting time values is associated with significantly less bleeding than heparin administration guided by the patient's body weight alone.
HARMONEE study initiated in Japan
OrbusNeich has initiated the HARMONEE study in Japan to evaluate the Combo dual therapy stent in patients presenting with ischaemic coronary disease and non-ST segment myocardial infarction.
OrbusNeich’s Combo Dual Therapy stent featured at JIM
Robbert de Winter, of the Academic Medical Centre, Amsterdam, The Netherlands, presented an update on the REMEDEE Registry, a post-market registry to evaluate the long-term safety and performance of the Combo Dual Therapy stent in routine clinical practice at the Joint Interventional Meeting.
First patient’s own stem cells administered in largest-ever trial of stem cell therapy in heart attack patients
The patients admitted to the London Chest Hospital will be among the first of 3,000 individuals involved in the Europe-wide trial to test whether administering a patient's own stem cells shortly after a heart attack will prolong life.
Pioneering heart operation performed for the first time in Leicester
In a UK first, experts from Glenfield Hospital have repaired a dysfunctional heart valve by inserting a tiny implant measuring just 23mm in size.
ORBIT II coronary data to be presented at 2014 Cardiovascular Research Technologies Conference
One-year data from the ORBIT II study of the Diamondback 360 coronary orbital atherectomy system in treating severely calcified lesions will be presented as an iMPACT Trial at the 2014 Cardiovascular Research Technologies conference in Washington, DC, USA.
New heart disease and stroke risk estimator App now available
The American College of Cardiology and the American Heart Association have released a mobile and web-based app for health care professionals to use with their patients in determining 10-year and lifetime risks for developing atherosclerotic cardiovascular disease.
Robotic-assisted coronary angioplasty procedures will be highlighted during CRT 2014
Corindus Vascular Robotics announced that cases submitted by users of its CorPath Vascular Robotics system have been accepted for presentation at the Cardiovascular Research Technologies (CRT) conference on February 22 – 25, 2014 in Washington, DC, USA.
US trial of Sentinel cerebral protection system approved
The SENTINEL trial to evaluate the safety and efficacy of the Sentinel cerebral protection system for embolic protection during Transcatheter Aortic Valve Implantation has received Investigational Device Exemption (IDE) approval from the Food and Drug Administration (FDA).
Cardiac Dimensions receives positive reimbursement decision for 120 hospitals across Germany
Cardiac Dimensions announced that it has received German Neue Untersuchungs und Behandlungsmethoden (NUB) Status 1 approval across 120 leading hospitals in Germany, which may now request reimbursement from their associated insurance companies to cover the costs of Carillon mitral contour system procedures.
Philips reaches enrolment goal in largest ever sleep apnea clinical trial
Royal Philips has reached its enrolment goal in the SAVE study, which examines the impact of leading obstructive sleep apnea treatment on cardiovascular disease.
Terumo receives CE mark for its new drug-eluting stent, Ultimaster
Terumo Corporation announced that it has received CE mark approval for its drug-eluting stent, "Ultimaster", with features expected to reduce stent thrombosis and to produce favourable long term clinical outcomes.
The use of vascular robotic systems for PCI
Ronald P Caputo writes about robotic systems for percutaneous coronary intervention designed to provide greater accuracy of lesion measurement, aid in better advancement of guidewires and help ensure correct placement of stents.
Surprising trends in cause of long-term death after percutaneous coronary intervention
More people who have known coronary heart disease die from other causes - such as cancer, and lung and neurological diseases - than heart disease, compared with 20 years ago, according to a Mayo Clinic study published online in Circulation, an American Heart Association journal.
Study finds obesity during pregnancy is independent risk factor for long-term cardiovascular morbidity
A recent study has found that obesity during pregnancy is an independent risk factor for long-term cardiovascular morbidity. Researchers concluded that obese pregnant patients might benefit from cardiovascular risk screening that could lead to early detection and secondary prevention of cardiovascular morbidity.
Global Genomics Group completes patient enrolment of discovery cohort in GLOBAL study ahead of schedule
Enrolment of the 5,000-patient discovery cohort of the GLOBAL (genetic loci and burden of atherosclerotic lesions) clinical study, designed to identify disease-related pathways, new drug targets and biomarkers for cardiovascular diseases, has been completed. Pilot data for the first cohort of patients are expected later this year.
FDA reviewer says no to cangrelor for PCI
In a clinical review evaluating cangrelor for reducing thrombotic cardiovascular events during percutaneous coronary intervention, one reviewer says the drug should not be recommended for this indication because studies have failed show that a cangrelor regimen is superior or non-inferior to a clopidogrel regimen or standard of care.
High sensitivity cardiac troponin T assay may lead to over diagnosis of myocardial infarction
A new study indicates using a uniform 14ng/L cutoff for the high sensitivity cardiac troponin T assay, rather than adapting the cutoff for the patient's age or gender, may lead to over diagnosis of myocardial infarction particularly in elderly men.
App for patients with elevated risk of cardiac events launched
CathMaps+ is the world’s first mobile application for people with an elevated risk of a cardiac incident, which integrates their cardiac history with an interactive map of catheterisation Labs throughout most of the world, and has now been launched in the USA.
Ashok Seth
Ashok Seth is the immediate past president of Cardiological Society of India and is the principal investigator of the Absorb First registry. He has been awarded the "Padma Shri" by the president of India, one of the highest civilian honours of the Government of India, and talks to Cardiovascular News about performing his father's angioplasty, his mission to make Delhi state a safer place to live, and the importance of planning ahead to avoid complications.
Managing mild paravalvular leak after TAVI
Paravalvular leak after transcatheter aortic valve implantation (TAVI) continues to be a concern, and the question of whether mild paravalvular leak is important remains unanswered. Susheel Kodali speaks to Cardiovascular News about the identification and management of mild paravalvular leak.
Pattern of higher blood pressure in early adulthood helps predict risk of atherosclerosis in middle-age
A study published recently in the Journal of the American Medical Association, reports that in an analysis of blood pressure patterns over a 25-year span from young adulthood to middle age, individuals who exhibited elevated and increasing blood pressure levels throughout this time period had greater odds of having higher measures of coronary artery calcification.
Symptoms of depression causally linked to risk of coronary heart disease in UK Whitehall study
A report published recently provides evidence that the symptoms of depressive disorder are causally associated with the risk of coronary heart disease, and as such should be considered a potentially modifiable risk factor for the occurrence of coronary heart disease.
Claret Medical announces CE mark and European product launch of the Sentinel cerebral protection system
Claret Medical has announced that it has received CE mark for the Sentinel cerebral protection system for embolic protection during transcatheter aortic valve implantation (TAVI). The product will launch immediately in selected CE mark countries.
Admedus CardioCel cleared for sale in the USA
Admedus has received Food and Drug Administration (FDA) clearance to market CardioCel in the USA. CardioCel will be used in pericardial closure and for the repair of cardiac and vascular defects in both adults and paediatrics.
Sapien XT can now be used for valve-in-valve procedures
Edwards Lifesciences has announced that it has received CE-mark approval for its transcatheter heart valve, Sapien XT, to be used for valve-in-valve procedures in both the aortic valve and the mitral valves.
PCI expansion speeds up treatment time
According to the latest UK national audit, the expansion in the use of PCI procedures has meant that patients with acute coronary syndromes are treated more quickly.
Neovasc announces first human implant of Tiara transcatheter mitral valve
Neovasc has announced that a first-in-human implantation of its Tiara transcatheter mitral valve was successfully performed on January 30th by physicians at St. Paul's Hospital in Vancouver.
Durability testing completed for Transcatheter Technologies’ prosthetic aortic heart valve
Transcatheter Technologies has announced it has completed durability testing for its protective aortic heart valve, which is an intrinsic part of the company's transcatheter aortic valve implantation (TAVI) device, Trinity.
Ultrasound renal denervation system receives CE mark
ReCor Medical has announced that it has received CE-mark approval for the latest generation of its ultrasound-based renal denervation system. It adds that the first patients were treated with the new system in December 2013 at the Universitäts–Herzzentrum, Bad Krozingen, Germany.
Majority of patients believe PCI cures their coronary artery disease
A survey has found that barely one in four patients who have undergone percutaneous coronary intervention know that they still have coronary artery disease as many patients believe that the procedure cures their condition.
Recurrent chest discomfort is the most common cause of readmission after PCI
A study published ahead of print in Circulation: Cardiovascular Interventions shows that the most common cause of readmission after percutaneous coronary intervention is recurrent chest discomfort. However, few patients with recurrent chest discomfort meet the criteria for myocardial infarction.
A smartphone app to assist valve-in-valve procedures
Vinayak Bapat writes about the key features of his app that provides advice on performing valve-in-valve procedures and explains why he feels such an app is necessary
Edwards Lifesciences launch US trial of Sapien 3 TAVI device in intermediate risk patients
Edwards Lifesciences has announced that it will begin a US trial of its Sapien 3 transcatheter aortic valve implantation (TAVI) device in patients who are at intermediate risk.
Royal Philips forms Healthcare Informatics Solutions and Services
Royal Philips has announced the formation of Healthcare Informatics Solutions and Services, a new business group within Philips' Healthcare sector that offers hospitals and health systems the customised clinical programmes, advanced data analytics and interoperable, cloud-based platforms necessary to implement new models of care.
First commercial implant of Elixir Medical’s DESolve takes place in Germany
Elixir Medical has announced that the first commercial implant of its novolimus-eluting bioresorbable coronary scaffold–DESolve has taken place in Germany. The device is designed to degrade within one year.
On-X Life Technologies receives approval for expanded labelling claim
On-X Life Technologies has announced today that its previously CE-marked On-X prosthetic heart valve has received European regulatory approval for an expanded labelling claim, which now permits the company to market its valve with a reduced requirement for the use of blood-thinning drugs such as warfarin.
Another blow for renal denervation as Covidien ends OneShot renal denervation programme
Covidien has announced that because of a slower than expected development of the renal denervation market, it is voluntarily exiting its OneShot renal denervation programme.
CoreValve approved in the USA
Medtronic has announced the FDA approval of its self-expanding transcatheter CoreValve system for severe aortic stenosis patients who are too ill or frail to have their aortic valves replaced through traditional open-heart surgery.
Pacemaker implantation after TAVI does not increase risk of all-cause mortality
A study published ahead of print in Circulation did not find a significant difference in the rate of all-cause mortality at two years between patients who received a permanent pacemaker within 30 days of undergoing TAVI and TAVI patients who did not receive a pacemaker.
Echocardiographic essentials for TAVI
Rebecca Hahn reviews the expanding role of echocardiography in transcatheter aortic valve implantation (TAVI), discussing the use of this imaging modality in treatment planning, intraprocedure guidance, and post-procedure assessment.
Barostim therapy may be cost-effective for resistant hypertension
A health economics analysis published in the Journal of Hypertension suggests that Barostim therapy is a cost-effective treatment option for patients with drug-resistant hypertension
CE mark for catheter that delivers biological therapies to the heart muscles
BioCardia has announced that it has received the CE mark for its Helix transendocardial delivery catheter, which has been optimised for enlarged ventricles and, therefore, increases the potential patient pool for transendocardial delivery of stem cell therapy.
First fully repositionable 29mm TAVI device gets CE mark
Direct Flow Medical has announced that it has received the CE mark for its fully repositionable 29mm transcatheter aortic heart valve, which is delivered through a flexible, 18-French transfemoral delivery system.
Stentys signs first distribution partnership for its stents in Asia
After establishing marketing in Europe and the Middle East, Stentys has announced it is continuing its international development by establishing partnerships with national distributors specialised in cardiovascular products in Singapore, Hong Kong and Malaysia.
CE mark clearance for MediValve’s acWire guidewire
A new guidewire (acWire, MediValve) has received CE mark clearance. The guidewire is intended to facilitate the accurate placement and alignment of medical devices in the cardiovascular system during diagnostic and interventional cardiovascular procedures.
Boston Scientific appoints new chief medical officer for interventional cardiology
Boston Scientific has appointed Craig Thompson as its new senior vice president chief medical officer of interventional cardiology. The company reports that Thompson will play a key role in driving the development of innovative medical solutions within the Boston Scientific interventional cardiology business.
Tryton Medical launches new Bifurcation Institute
According to the company, the new institute is a comprehensive resource that provides education and training for physicians treating bifurcated coronary artery disease
EMA accepts marketing authorisation application for cangrelor
The Medicines Company has announced that the European Medicines Agency (EMA) has accepted for review a marketing authorisation application for the investigational intravenous antiplatelet agent cangrelor.
ACC launches criteria for evaluating stable ischaemic heart disease tests
The American College of Cardiology, along with nine key specialty and subspecialty societies, has published a document assessing 80 potential clinical scenarios with the goal of assisting physician and patient decision-making.
CE mark approval expands patient group for Portico TAVI system
St Jude Medical has received the CE mark for its 25mm transcatheter aortic valve implantation Portico device, meaning that more patients can now undergo TAVI with the Portico system.
Boston Scientific and The Medicines Company will co-promote Promus Premier in USA
Boston Scientific and The Medicines Company have announced a co-promotion agreement for the Promus Premier stent system. Therefore, The Medicines Company's acute cardiovascular care sales force will collaborate with the Boston Scientific Interventional Cardiology sales force to provide promotional support for the stent system in USA hospitals beginning January 2014.
First mobile CT scanner launched in UK
Toshiba Medical has announced that it has become the first medical equipment provider to offer a mobile CT scanner service in the UK. The state-of-the-art Aquilion CXL scanner, a high-end, low dose and fast reconstruction CT scanner was launched at the start of December.
Impaired cardiac baroreflex sensitivity predicts response to renal sympathetic denervation
Study author Axel Bauer describes the main findings of a study that investigates whether impaired cardiac baroreflex sensitivity could be used to identify patients who will respond to renal denervation. The study was published ahead of print in the Journal of the American College of Cardiology.
Sutureless aortic valve replacement could be first-line therapy for grey-area patients
A study comparing transcatheter aortic valve implantation with minimally invasive aortic valve replacement with a sutureless valve has found that the latter approach is associated with less paravalvular leak, reduced mortality and could possibly be a first-line treatment for (grey-area) patients who fall between surgery and TAVI.
Rick Olson named as new vice president of global sales operations at InspireMD
InspireMD has announced that it has appointed Rick Olson, who was previously the director of international strategy and high performance management system at Covidien, as its new vice president of global sales operations.
Global renal denervation market will be worth $171.7m by 2019
According to GlobalData, the global renal denervation market will significantly increase from $15.5m (its 2012 level) to $171.7m by 2019. The research and consulting firm say that the expected increase will be a result of the intervention's approval in major countries.
Meta-analysis raises questions about ischaemia-driven revascularisation
A meta-analysis indicates that percutaneous coronary intervention does not reduce the risk of death, non-fatal myocardial infarction, unplanned revascularisation, or angina compared with medical management alone in patients with stable coronary artery disease and myocardial ischaemia.
CE mark for new Symplicity system
Medtronic has announced that it has received both CE-mark approval and Australian therapeutic goods administration (TGA) listing for a new Symplicity renal denervation system, consisting of a flexible 4 French multielectrode catheter (Symplicity Spyral) and a radio frequency generator (Symplicity G3).
HeartWare acquires Circulite
HeartWare has announced that it has acquired CircuLite, which developed the Synergy circulation support system for the management of less sick, ambulatory, chronic heart failure patients who are not yet inotrope-dependent.
Siemens launches Artis one angiography system for universal use
At the Radiological Society of North America annual meeting (1–6 December, Chicago, USA), Siemens will launch its new Artis one angiography system for universal use. The new system has been optimised for broad clinical use.
Successful 30-day results for truly repositionable TAVI system
A pilot study of a "truly prepositional" transcatheter aortic valve implantation (TAVI) system indicates that the system (Trinity, Transcatheter Technologies) is associated with reduction in mean pressure gradient from 59mmHg at baseline to 20mmHG at 30 days post-implantation.
Esaote showcases its prevention suite at EuroEcho 2013
Esaote has announced it will demonstrate its prevention suite, a combination of four imaging technologies that enables practical pre-clinical assessment of cardiovascular disease, at the EuroEcho-Imaging conference in Istanbul, Turkey (11-14 December)
New document on transcatheter therapies for mitral regurgitation launched
The American College of Cardiology, the American Association for Thoracic Surgery (AATS) the Society for Cardiovascular Angiography and Interventions (SCAI), and The Society of Thoracic Surgeons (STS) have published a joint document on using transcatheter therapies to manage mitral regurgitation
FDA approves Boston Scientific’s Promus Premier coronary stent
According to a company release, the Promus Premier stent features customised platinum chromium alloy stent architecture, an everolimus drug with a biocompatible, fluorinated co-polymer, and an enhanced stent-delivery system.
DAPT and DES in coronary artery disease: Is it time to revisit the guidelines? – Part 1
Alexandre Abizaid, Azeem Latib and Sigmund Silber discuss whether the current US and European dual antiplatelet therapy guidelines for patients treated with drug-eluting stents should be revisited. Flavio Ribichini moderates the discussion. In the first part of this webcast, Abizaid presents the past and current guidelines.
DAPT and DES in coronary artery disease: Is it time to revisit the guidelines? – Part 2
In the second part of the webcast, Latib presents data demonstrating that DAPT longer than 12 months increases the risk of bleeding; he also talks about differences between first and second generation DES and their impact on DAPT duration. Silber speaks about the results of the RESOLUTE clinical programme.
CE mark for XL version of Perceval surgical aortic valve
Sorin has announced that it has received the CE mark for the XL version of its Perceval bioprosthetic aortic valve, which is designed to replace a diseased native or malfunctioning prosthetic aortic valve using either traditional or minimally invasive heart surgery.
First implant of new Solo Smart stentless aortic valve
After receiving CE mark approval, Sorin's Solo Smart biological aortic pericardial heart valve has been implanted for the first time. The bioprosthesis is designed to respect the aortic root physiology and ensure a physiological blood flow through the valve annulus.
Alvimedica and CID join forces
Alvimedica has announced a merger with CID (Carbostent & Implantable Devices). Both companies will now work under the brand name Alvimedica.
Enrolment completed in DIRECT II study of rapamycin-eluting stents with biodegradable polymer
Svelte Medical Systems has announced that enrolment in its DIRECT II study, which is assessing the use of its rapamycin-eluting stent with a biodegradable polymer in patients with coronary artery disease, has been completed.
Lotus TAVI device implanted for the first time in Europe
After receiving CE mark approval, the Lotus transcatheter aortic valve implantation (TAVI) device has been implanted in two German patients.
Campless technique may minimise strokes during CABG
A study has indicated that a campless technique, consisting of off-pump coronary artery bypass in combination with the Heartstring proximal seal system, reduces the risk of perioperative strokes during coronary artery bypass grafting (CABG).
Drug-eluting stents reduce target vessel revascularisation in older patients
The results of the XIMA (Xience or Vision stents for the management of angina in the elderly) study indicates that in patients aged ≥80 years, the use of drug-eluting stents is associated less myocardial infarction and target vessel revascularisation than bare metal stents.