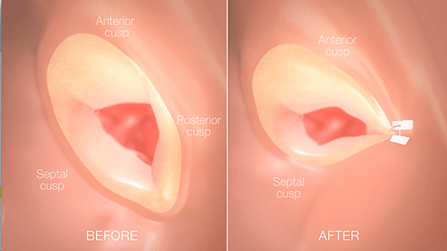
Mitralign has raised US$39.8 million to date in a Series E equity round of financing. With the Series E financing raised, the company plans to pursue US and CE regulatory approval for the commercialisation of their Trialign system, in parallel with preparations for European commercial launch of their Mitralign percutaneous annuloplasty system (MPAS).
“The capital will be used to accelerate clinical trials both in the USA and in Europe for the Trialign System and support our leadership in the tricuspid space” says Rick Geoffrion, chief executive officer of Mitralign. “We look forward to the approaching completion of the SCOUT early feasibility study in the USA and the initiation of the SCOUT II study to support the CE mark in Europe as we expand our tricuspid clinical data set.”
Both the Trialign and MPAS systems feature a customisable therapy solution with an extremely small footprint, designed to leave all clinical options open for the physician. The Trialign system is currently enrolling patients in an early feasibility IDE study in the US and is not approved for sale or distribution. The MPAS received CE mark approval in February for the treatment of functional mitral regurgitation (FMR).











