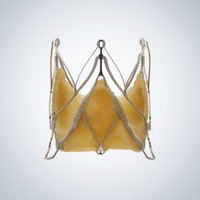
JenaValve Technology has received EN ISO 13485:2012 certification for its manufacturing facility in Leeds, United Kingdom.
According to a press release, this certification covers the design, development, manufacturing and distribution of JenaValve’s transcatheter heart valves.
Victoria Carr-Brendel, chief executive officer of JenaValve says, “Going forward, this facility will supply the valves needed to support our clinical trial programs and future commercial launches.”
The ISO (International Organization for Standardization) is the world’s largest developer and publisher of International Standards. JenaValve’s EN ISO 13485:2012 + AC: 2012 certificate was awarded by the notified body, DEKRA.










