The FDA has approved Medtronic’s CoreValve Evolut Pro transcatheter aortic valve implantation (TAVI) device. This approval follows data for the device that were presented at the 2017 scientific sessions of the American College of Cardiology (ACC; 17–19 March, Washington, DC, USA); these 30-day clinical data, a press release reports, show that CoreValve Evolut Pro is associated with high survival, low rates of stroke, minimal paravalvular leak and excellent haemodynamics.
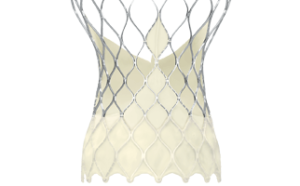
The press release states that the Evolut Pro device features a “unique valve design” with an outer wrap that adds surface area contact between the valve and the native aortic annulus to further advance valve sealing performance. The biocompatible porcine pericardial tissue wrap, in addition to other design elements, is incorporated to address the occurrence of blood leaking through the sides of the valve.
The Evolut Pro clinical study (N=60) met its primary endpoint at 30 days with high rates of survival (98.3%) and low rates of disabling stroke (1.7%). The Evolut Pro valve also showed strong haemodynamic performance with large aortic valve areas (2± 0.5cm2) and mean gradients in the single digits (6.4±2.1mmHg) at 30 days. The majority of study patients (72.4%) experienced no/trace paravalvular leak and no incidents of moderate or severe paravalvular leak were observed at 30 days. Additionally, the rate of new pacemaker implantation was 10%.
John Forrest (Yale University School of Medicine, New Haven, USA, who presented the data at the ACC, says: “The 30-day clinical outcomes presented at ACC demonstrate the Evolut Pro valve to be an outstanding treatment option for patients with severe aortic stenosis who are at a high or extreme risk for surgery.”
The 23 mm, 26mm and 29mm sizes of the Evolut Pro system are available for use in the USA and the valves for patients who are inoperable or at high risk. It is not available for use in countries outside of the USA.












