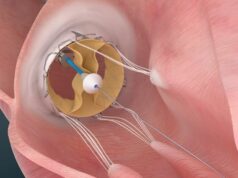
NaviGate Cardiac Structures has announced that the Gate catheter-guided tricuspid atrioventricular valved stent was implanted through the jugular vein one week ago in a patient with severe tricuspid regurgitation stemming from two failed tricuspid annuloplasty rings that were unable to maintain the patient’s valve competence.
The patient, a 78-year-old man with a long history of cardiac conditions, has undergone four cardiac surgeries, including two coronary bypass surgeries and two failed valve repairs. The patient had become symptomatic of right heart failure and opted to make a compassionate plea to his physician for the valved stent under development. The FDA allowed the compassionate use. Jose Navia and Samir Kapadia proceeded with the implantation. After receiving the valve, the patient became stable. He has been closely watched by the cardiac team and was discharged to home.
Navia, the staff surgeon and researcher who sponsored the use at Cleveland Clinic (USA), says: “We were able to perform this procedure in its entirety percutaneously through the jugular vein in a beating heart. In addition, the tricuspid valve was implanted within the failed annuloplasty ring, providing another alternative in treating tricuspid disease.” He is a member of NaviGate’s scientific advisory board and a company shareholder. As the inventor of the technology, he is entitled to a portion of any commercialisation revenues received by Cleveland Clinic.
The Gate tricuspid atrioventricular valved stent has been developed and manufactured by NaviGate Cardiac Structures. The company also has developed the percutaneous “NAVI” valve for mitral valve replacement as well as tissue-preservation techniques that remove the toxic tissue fixatives thought to affect the longevity of tissue. This technology removes most of the liquid so that the device can be shipped in the “dry” form. The company licensed the seminal technology from Cleveland Clinic.
NCSI made modifications to the device that differentiates it from all others presently manufactured for atrioventricular valves. The unique design of this device in the form of a diffuser or truncated cone exhibits a low-height profile that can be more easily threaded through the vasculature to reach the atrioventricular valves, allowing it to reside without protrusion into either of the adjacent chambers (atrium or ventricle) for mitral or tricuspid valves, while simultaneously allowing the largest possible diameters to capture the enlarged annulus typical of the dysfunctional tricuspid valve resulting from Dilated Cardiomyopathy (DCM); thus, valves in sizes of up to 48—50mm can be manufactured.