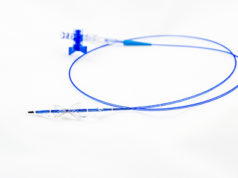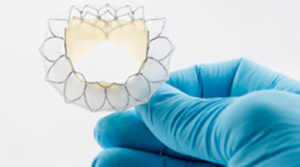
Neovasc has provided an update on the study progress and clinical performance of the Tiara valve, a self-expanding mitral bioprosthesis for transcatheter implantation in patients with mitral regurgitation. According to a press release, to date, 34 patients have been treated with the Tiara valve at 10 different medical centres across the USA Canada, Germany, Italy, Belgium, Switzerland and Israel.
The press release reports that the technical success rate in these implantations is 31/34 or 91.1%. In these technically successful implantations, paravalvular leak levels were reported as mild, trace or absent in 100% of these cases. All cause, 30-day mortality in the 33 patients who have reached 30 days post implant with Tiara is 12.1% (4/33). The remaining patient treated within the last 30 days is recovering well. To date, the longest surviving patient has passed 3.5 years post implant.
Ulrich Schafer (Cardiology Department at the University Heart Center Hamburg, Hamburg, Germany), one of the CE Mark trial’s principal investigators, says: “Transcatheter implantation of the Tiara mitral valve resulted in immediate elimination of mitral regurgitation and improved the performance of the heart, without the need for any cardiac support device and with no procedural complications. The results we see so far are very encouraging in this very sick and high-risk patient population. These patients with severe mitral regurgitation and severe heart failure tolerate the Tiara implantation procedure without any major issues, and most of them are discharged home three to five days after the valve implantation.”
Implantations of the Tiara are being performed under three parallel clinical/investigational programs:
- A European pivotal CE Mark trial, TIARA-II
- An FDA Early Feasibility trial, TIARA-I
- Compassionate use/special access treatment.
To date, the Neovasc has received regulatory approval in Italy, Germany and the UK to conduct the TIARA-II study at 10 centres (five in Italy, three in Germany and 2 in the UK). The TIARA-II study, which is the primary focus of the Tiara programme, is a 115 patient, non-randomised, prospective clinical study evaluating Tiara’s safety and performance. It is expected that data from this study will be used to file for CE mark approval.
Additionally, the company has recruited two new US centres to participate in its TIARA-I study, and is actively recruiting in four centres in the USA, one centre in Belgium, and three centres in Canada. TIARA-I is an international, multicentre early feasibility study being conducted to assess the safety and performance of the Tiara mitral valve system and implantation procedure in high-risk surgical patients suffering from severe mitral regurgitation.