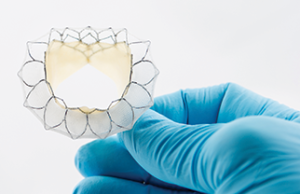
Tag: Tiara
TMVI with Tiara valve may be safe and feasible for patients with prior aortic valve replacement
A new study indicates that transcatheter mitral valve implantation (TMVI) with the Tiara valve (...
TVT 2018: Tiara TMVI device featured in live case
Neovasc announced that its Tiara transcatheter mitral valve implantation (TMVI) device was featu...
Neovasc provides Tiara clinical update
Neovasc has provided an update on the study progress and clinical performance of the Tiara valve...
German court awards Edwards Lifesciences co-entitlement of Neovasc’s Tiara patent application
The District Court in Munich, Germany has partially found in favour of Edwards Lifesciences Corp...
Boston Scientific closes acquisition of certain Neovasc assets
Boston Scientific Corporation has closed its acquisition of certain manufacturing assets and cap...
Neovasc receives approval to launch European study of Tiara transcatheter mitral valve
Neovasc has received both regulatory and ethics committee approval to initiate the Tiara transca...
TCT 2016: Tiara TMVI device highlighted in presentations
Neovasc has announced today its notable presentations at the 2016 Transcatheter Cardiovascular T...
FDA gives Neovasc go-ahead to add 40mm valve size to its TIARA-I clinical trial
The FDA has given Neovasc approval for participating physicians to treat patients with the c...