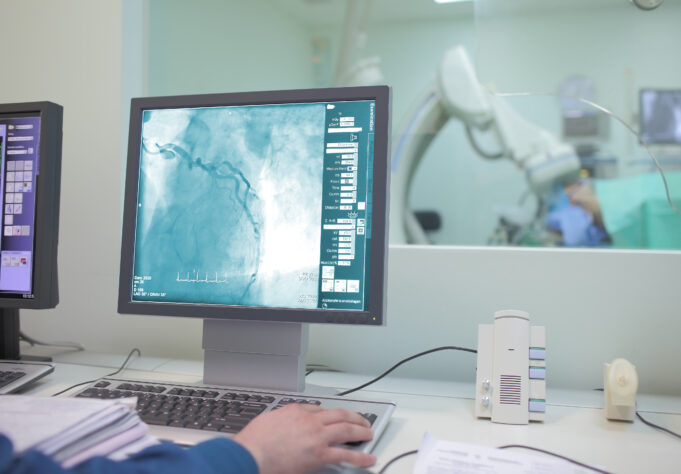
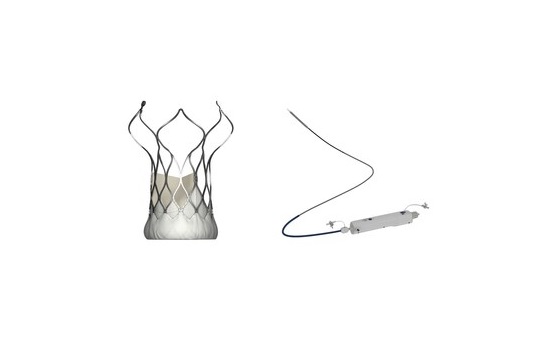
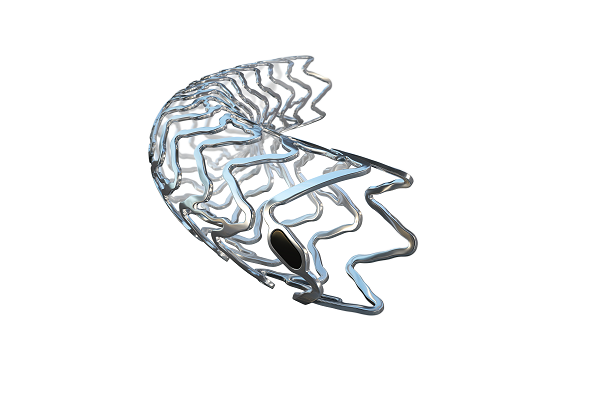
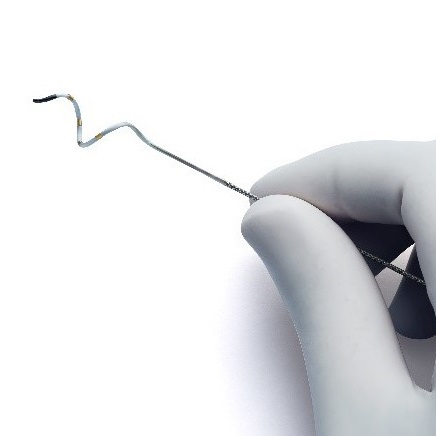
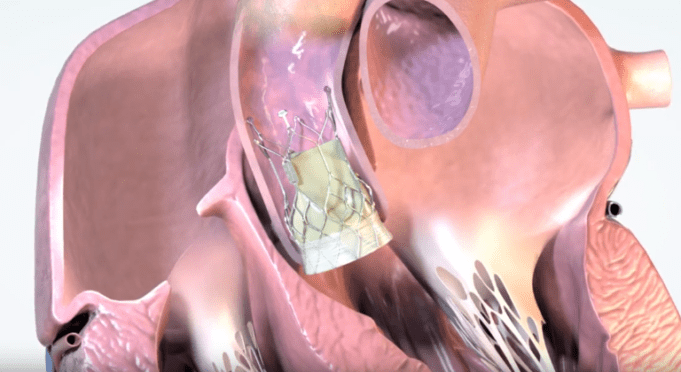
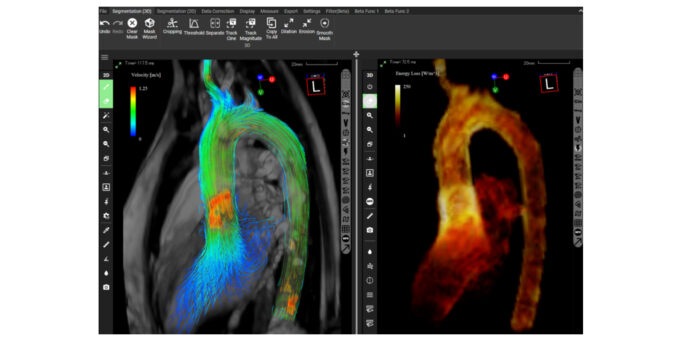
The UK’s National Institute for Health and Care Excellence (NICE) has today publ...
Transcatheter therapies for the treatment of tricuspid regurgitation (TR) have g...
CorWave has announced the first-in-human implantation of its left ventricular as...
CorFlow Therapeutics has announced that the US Food & Drug Administratio...
Merit Medical recently announced the appointment of Martha Aronson as the compan...
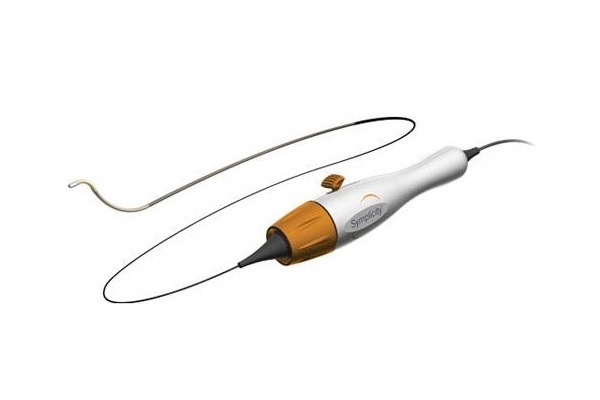
Medtronic has announced that the treatment of the first patient in the SPYRAL GE...
Medtronic has announced that its VitalFlow extracorporeal membrane oxygenati...
New analysis of the EARLY TAVR trial, in which investigators examined the preval...
The first US procedures have been performed in the FRACTURE investigational ...
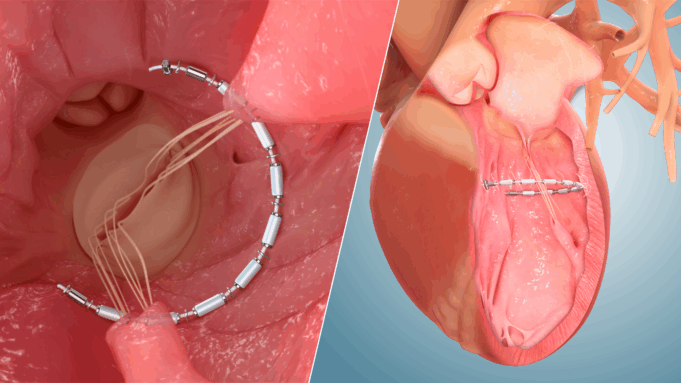
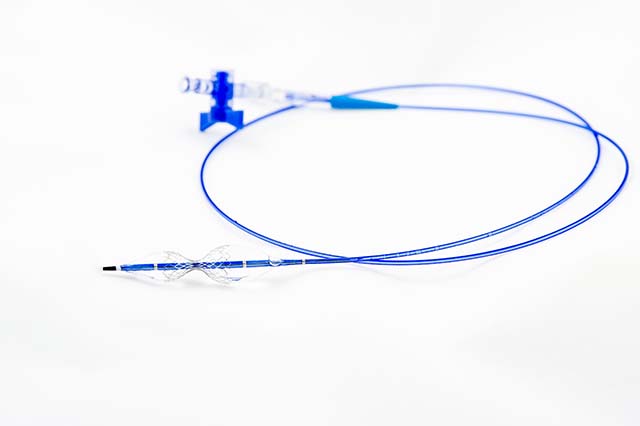
CroíValve, developer of the Duo transcatheter system for the treatment of tricus...
Thirty-day results of the US early feasibility study assessing the use of th...
Degenerative mitral valve disease has doubled in prevalence globally over th...
Teleflex today announced that is has completed the previously announced acquisit...
InterShunt Technologies has announced the start of the EASE HF2 early feasibilit...
Foldax has announced the presentation of one-year results from the India cli...
Analysis of real-world US data comparing outcomes after post-transcatheter aorti...
Cardiosense has announced the publication of results from its SEISMIC-HF I s...
Vivasure Medical has announced the submission of a premarket approval (PMA) ...
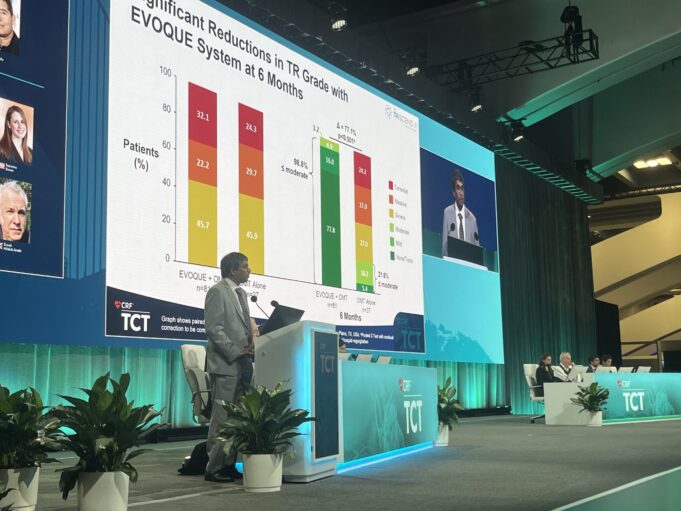
Edwards Lifesciences has announced that the company’s Evoque tricuspid valve...
AngioDynamics has announced the first patient has been enrolled in the RECOVER-A...
Ancora Heart has announced that it has reached the first enrolment milestone...
Research presented at the American Diabetes Association (ADA) 2025 scientific se...
The results of a retrospective analysis should act as a “word of caution” ag...
Long-term results of the UK TAVI trial, a pragmatic trial comparing transcat...
Ceryx Medical has announced the successful closure of a new funding round, bring...
New regulations have come into effect in the UK which place a greater emphasis o...
Prevencio has been granted patents for its HART CADhs test in the USA—the second...
Adona Medical has announced the completion of enrolment in the ATHENS-HF fir...
MedHub-AI has announced a distribution agreement with Terumo Corporation to ...
Corvia Medical has announced the successful closure of a US$55 million fundi...
Empagliflozin preserved kidney function and was safe to initiate in heart at...
Inquis Medical has announced that its Aventus thrombectomy system has received 5...
Penumbra has announced the completion of enrolment in the STORM-PE clinical ...
Enrolment has been completed in the TRANSFORM II randomised controlled trial, co...
Atraverse Medical has announced the close of US$29.4 million in follow-on financ...
Jenscare has announced the release of 30-day follow-up results of the TRINIT...
Medicare patients with atrial fibrillation (AF) who undergo surgical ablation du...
Foldax has announced that the Indian Central Drugs Standard Control Organization...
Biotronik has announced the enrolment of the first patient in the Leave Nothing ...
FastWave Medical has announced the successful completion of initial first-in...
Boston Scientific has announced the surprise move to cease sales of its Acurate ...
New data comparing the Myval transcatheter aortic valve implantation (TAVI) ...
Abbott has announced US Food and Drug Administration (FDA) approval for its ...
Medtronic has received CE mark approval for several expanded indications for the...
TRiCares has announced the presentation of data in a late-breaking session a...
Elixir Medical has announced three-year results from the 445-patient BIOADAP...
Thirty-six-month follow-up data from the BIOMAG-I first-in-human trial assessing...
Medtronic has announced it has received CE mark for the expanded redo transc...
Two-year outcomes from the bRIGHT registry, a prospective multicentre regist...
Transcatheter aortic valve implantation (TAVI) can be a lifesaving intervention ...
A pooled, individual patient-level data meta-analysis of two trials investigatin...
Royal Philips has announced the launch of the RADIQAL trial, a multicentre, ...
Thirty-day primary endpoint results of EMPOWER CAD, the first prospective, r...
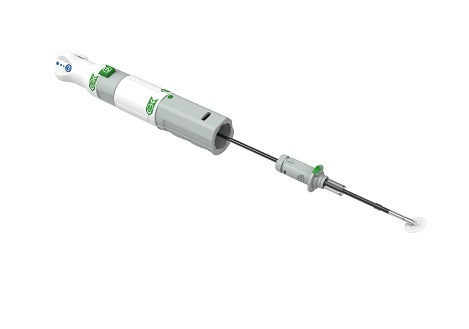
Cordis has announced the initiation of the SELUTION global coronary registry, a ...
Royal Philips has today announced the introduction of its VeriSight Pro 3D i...
Cytokinetics has announced positive topline results from MAPLE-HCM, a phase 3 cl...
New findings from the FAVOR III China trial demonstrate that kidney function...
With an increasing focus on the harmful impact of radiation exposure, as wel...
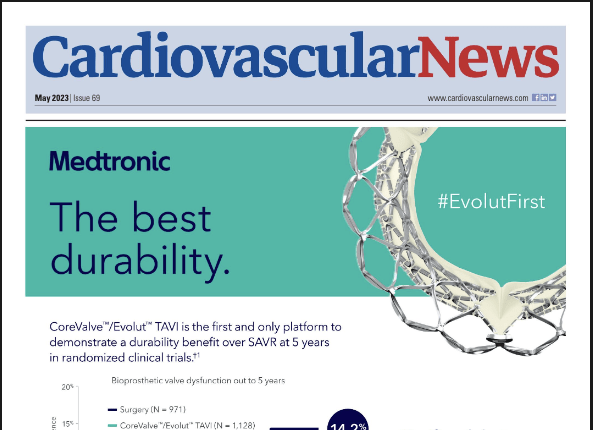
This advertorial is sponsored by Medtronic
As transcatheter aortic valve ...
Mayra Guerrero (Mayo Clinic, Rochester, USA) speaks to Cardiovascular News about...
In this issue:
Lancet Commission urges reframing of coronary disease toward...
Endospan recently announced the presentation of 30-day results from the stat...
PlaqueTec has announced new research findings from interim data analysis of its ...
Heartflow has announced two-year data from FISH&CHIPS, a real-world, mul...
Inquis Medical recently announced results from its AVENTUS trial evaluating the ...
FastWave Medical has secured institutional review board (IRB) approval to commen...
The Society for Cardiovascular Angiography & Interventions (SCAI) has an...
Analysis from the EARLY TAVR trial, presented at the 2025 Society for Cardiovasc...
A novel technique to reduce left ventricular outflow tract (LVOT) obstructio...
In patients undergoing chronic total occlusion (CTO) percutaneous coronary inter...
Single antiplatelet therapy (SAPT) after transcatheter aortic valve implantation...
Results from the Central blood pressure and variability evaluation in abdomi...
In Wednesday's peripheral arterial programme, which hosted several podium-first ...
Edwards Lifesciences has announced that the US Food and Drug Administration ...
Orchestra BioMed Holdings has announced that the US Food and Drug Administration...
The Society of Thoracic Surgeons (STS) has announced the launch of its latest su...
SoloPace Incorporated has announced both US Food and Drug Administration (FDA) c...
Transverse Medical has announced the completion of the first series of patients ...
Vivasure Medical has announced European CE mark approval of the PerQseal Elite v...
Orchestra BioMed Holdings has announced that the US Food and Drug Administration...
Edwards Lifesciences has announced new eight-year data showing that patients rec...
Terumo Health Outcomes (THO), a division of Terumo Interventional Systems (TIS),...
Edwards Lifesciences has announced that the company’s Sapien M3 mitral valve...
Five-year results of the FAME 3 trial, comparing fractional flow reserve (FF...
AtriCure has announced the first use of the newest AtriClip device for minimally...
Elixir Medical has announced EU medical device regulation (MDR) CE mark approval...
Four-factor prothrombin complex concentrate (4F-PCC)—a shelf-stable medicati...
A STRIKE-PE interim analysis of clinical outcomes of acute pulmonary embolis...
Results of the Altavalve early feasibility study (EFS) evaluating outcomes o...
HighLife SAS has announced that that the US Food and Drug Administration (FDA) h...
The US Food and Drug Administration (FDA) has cleared Agitated Solutions Incorpo...
Shockwave Medical, part of Johnson & Johnson MedTech, has initiated its ...
Pacemaker implantation within 30 days after a transcatheter aortic valve imp...
Anteris Technologies has announced that the biomimetic DurAVR transcatheter ...
Low-dose colchicine (0.5mg per day) significantly reduced the total plaque volum...
Conformal Medical has announced the enrolment of its 500th patient in the CO...
Experts in the treatment of coronary artery disease have recommended reframi...
Teleflex has announced US Food and Drug Administration (FDA) 510(k) clearance of...
Using intravascular imaging (IVI) to guide drug-eluting stent implantation d...
Patients undergoing transcatheter edge-to-edge repair (TEER) using the Tricl...
Edwards Lifesciences has announced new scientific evidence presented during ...
MiRus has announced the launch of the US multicentre early feasibility study...
Data from 500 patients treated for aortic regurgitation with the Trilogy (Je...
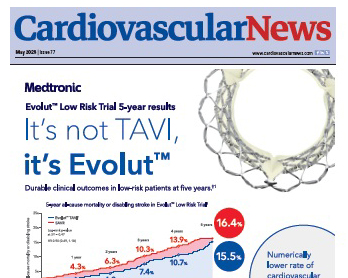
Latest data from the Evolut Low Risk trial demonstrate a numerically lower r...
Use of cerebral embolic protection with the Sentinel (Boston Scientific) dev...
Semaglutide significantly improved maximal walking distance in people with s...
An investigational monoclonal antibody called bentracimab can safely and eff...
At three months of follow-up, patients hospitalised for a serious heart atta...
Teleflex has announced that the preliminary results from its Ringer perfusion ba...
New data on the outcomes of transcatheter aortic valve-in-surgical aortic va...
Supira Medical has announced the successful completion of an oversubscribed seri...
Powerful Medical has been granted breakthrough device designation by the US Food...
Evident Vascular has announced the successful closing of its Series B financing ...
Abbott has announced that the US Food and Drug Administration (FDA) has appr...
Cardiac Dimensions has closed of a US$53 million series E financing round le...
Details of the successful first-in-human cases of robotic-assisted transcath...
GE HealthCare has announced the launch of the AltiX AI.i edition of its Mac-Lab,...
AngioInsight has announced the upcoming launch of its pivotal clinical study, SM...
Protembis has announced establishment of a scientific advisory board (SAB) t...
Consistent results have been seen out to two years in the AGENT investigatio...
Robert Wood Johnson University Hospital (RWJUH) and Rutgers Robert Wood John...
Laplace Interventional has completed its series C financing, which will be u...
Valcare Medical has announced the US Food and Drug Administration (FDA) inve...
Observational data from Sweden’s national registry on coronary and valvular ...
Best Cardiovascular Product Launch – Aurora Extravascular Implantable Card...
One of interventional cardiology’s master operators, known globally for his land...
Affluent Medical has appointed Howard Herrmann (Perelman School of Medicine ...
A large single-centre randomised trial has shown that the use of a protectiv...
Two-year results of the SMART trial, comparing two commonly used transcathet...
A real-world study of the Prevail (Medtronic) drug-coated balloon (DCB), com...
The Cardiovascular Research Foundation (CRF) has announced the appointment o...
Reflow Medical has announced the opening of its European subsidiary in Landsberg...
4C Medical Technologies has closed a US$175 million series D financing round, le...
A survey conducted by the Society for Cardiovascular Angiography and Interve...
Boston Scientific, Ajax Health and KKR have collaborated to create FlowMod, ...
Today, Shockwave Medical announced the US launch of its Shockwave Javelin pe...
Cadrenal Therapeutics has announced the signing of a collaboration agreement...
Stereotaxis has announced a US Food and Drug Administration (FDA) regulatory sub...
DeepQure has announced progress of clinical trials of its extravascular rena...
Boston Scientific has entered into a definitive agreement to acquire SoniVie...
Pi-Cardia has announced the first commercial procedures with its ShortCut de...
Pulnovo Medical has announced the closing of nearly US$100 million series C fina...
“This technique is here to stay,” says Raban Jeger (Basel, Switzerland) refl...
MyOme has announced the launch of its coronary artery disease (CAD) polygenic ri...
The American College of Cardiology (ACC) and the American Heart Association ...
Teleflex has today announced it has entered into a definitive agreement to acqui...
Biotronik is partnering with Egg Medical to co-sell Egg Medical’s EggNest radiat...
Medtronic has announced that the first patient has been enrolled in its pivotal ...
GE HealthCare has delivered the first patient doses of Flyrcado (flurpiridaz...
The Society for Cardiovascular Angiography & Interventions (SCAI) has publis...
Researchers have demonstrated the potential of a new drug—ataciguat—to signi...
In this issue:
World-first cases usher in new era for robotic cardiac surge...
In this issue:
World-first cases usher in new era for robotic cardiac surge...
This advertorial is sponsored by B Braun Melsungen AG
Drug-coated balloons (D...
iVascular has announced positive five-year results of the RANGO registry, demon...
Biotronik has announced the launch of BIOMAG-LL, a pre-market trial focused on c...
Despite a complicated early path into medicine, which was shaped by a major trag...
Sotagliflozin, a recently-US Food and Drug Administration (FDA) approved dru...
Critical challenges in open and endovascular treatment of aortic disease wil...
Avertix Medical has announced the publication of new data on the cost-effectiven...
WhiteSwell has announced positive data from 21 patients treated in the DELTA-HF ...
Orchestra BioMed has announced a late-breaking presentation of data on the b...
A new guideline from the American Society of Echocardiography (ASE) aims to prov...
Reprieve Cardiovascular has announced topline results from its FASTR randomised ...
Coronary computed tomography angiography (CCTA)-guided management of patient...
Two trials delivered in late 2024 have offered fresh evidence on the potential r...
A large-scale analysis of data from the US Centers for Medicare and Medicaid...
In this video interview, Cardiovascular News sits down with Bernardo Cortese...
Pending the results of several trials and “iterative changes” to device desi...
First-in-human results of a novel transseptal puncture device from Protaryx ...
A study assessing the performance of two commonly used definitions for determini...
CBM Lifemotion has announced Medical Device Regulation (MDR) CE mark certificati...
Amplitude Vascular Systems (AVS) recently announced that it has completed a Seri...
Artivion has announced data from the AMDS PERSEVERE clinical trial presented in ...
A new outreach programme by the European Association for Cardio-Thoracic Surgery...
In patients undergoing coronary artery bypass grafting (CABG), a novel analysis ...
The Society of Thoracic Surgeons (STS) has announced the election of Joseph Sabi...
Mechanical aortic valve replacement (AVR) provides significant long-term sur...
Clinical evidence supporting the efficacy, safety and efficiency of Atravers...
HeartFlow has partnered with the Boone Heart Institute (Colorado, USA) to launch...
Vantis Vascular has announced the first commercial use of its CrossFAST integrat...
Research published in the Journal of Cardiothoracic and Vascular Anesthesia,...
Caranx Medical has announced that it has made a submission to the US Food an...
Researchers from Mass General Brigham (Boston, USA) have evaluated a drug that r...
FastWave Medical has announced the issuance of its seventh utility patent by...
Humacyte has announced that it plans to file an investigational new drug (IND) a...
The US Food and Drug Administration (FDA) has issued a statement outlining m...
Taho Pharmaceuticals has announced the completion of the pivotal trial for TAH33...
Advanced Bifurcation Systems has announced the successful closure of a US$20.8 m...
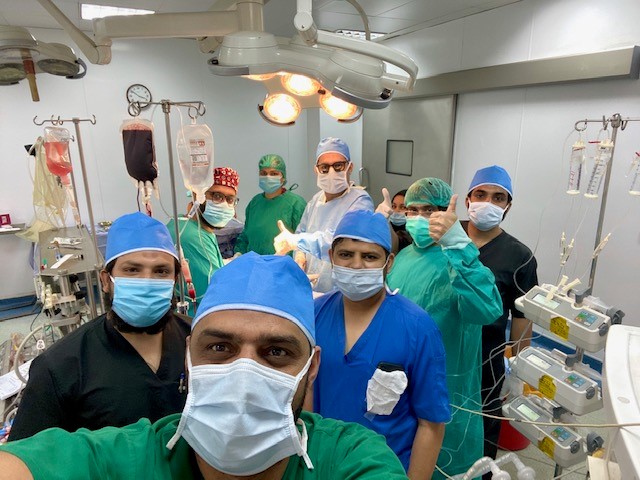
King Faisal Specialist Hospital and Research Centre (KFSHRC) in Riyadh, Saud...
Circa Scientific has announced the launch of its innovative Crosswise radiof...
Four cardiac surgery societies have endorsed recommendations of the European Soc...
Alleviant Medical has announced a US$90 million financing to fund its second piv...
The Centers for Medicare & Medicaid Services (CMS) is opening a national...
Biotronik has announced CE mark approval of two new indications for the Orsiro M...
CoreMedic has entered into a strategic partnership with InnoRa to accelerate cli...
AngioDynamics has announced the publication of the results from the Acute Pulmon...
Inquis Medical has announced completion of patient enrolment in its AVENTUS clin...
Piccolo Medical has announced an exclusive distribution agreement with Spect...
Argon Medical announces the first patient enrolment in the CLEAN-PE study. The p...
TRiCares has announced that the first patients have been successfully implan...
Relief Cardiovascular has announced the closing of a US$12 million series A fina...
Boston Scientific has announced it has entered into a definitive agreement t...
A recent analysis of over 270,000 Medicare fee-for-service beneficiaries has...
Simpson Interventions has announced that the Acolyte image-guided crossing and r...
Alleviant Medical has announced two milestones from the US Food and Drug Adminis...
The US Food and Drug Administration (FDA) has issued draft guidance that include...
Medtronic has received CE mark for the Harmony transcatheter pulmonary valve (TP...
BrosMed Medical has obtained CE mark for the RevoEdge high-pressure coronary cut...
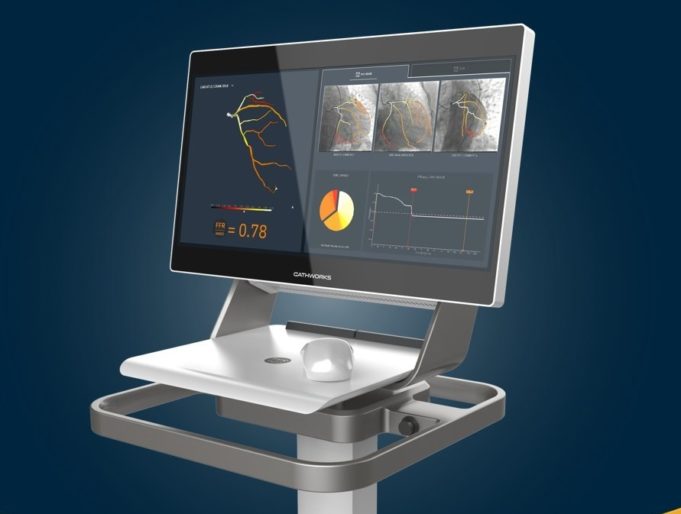
CathWorks has announced the successful completion of enrolment of the Advanc...
A trial comparing an invasive and a conservative strategy to treat patients ...
Which stories, features and interviews captured the attention of the cardiovascu...
Diabetes is one of the strongest risk factors for coronary disease, Pedro Le...
Reprieve Cardiovascular has announced the first-in-human results of the Reprieve...
BiVACOR has announced the successful completion of the first phase of the US Foo...
A randomised trial testing whether the removal of fasting requirements prior...
Performing percutaneous coronary intervention (PCI) significantly improved o...
JenaValve Technology has announced the completion of the first patient procedure...
FastWave Medical has announced the close of a US$19 million funding round ea...
The US Food and Drug Administration (FDA) has expanded the indications for the I...
Vantis Vascular has announced the successful closing of US$10 million series B-1...
Capstan Medical has announced the successful closing of an oversubscribed US$110...
The initial shortlist for the second Global Cardiovascular Awards—which will rec...
Protaryx Medical has announced the completion of its first-in-human (FIH) study,...
The rapid growth of transcatheter aortic valve implantation (TAVI) has been the ...
Terumo Health Outcomes (THO) and Medis Medical Imaging have entered into a strat...
New research suggests that cryoablation is a safe and effective approach to clos...
Live cases broadcast at interventional cardiology meetings are performed wit...
Seven-year results of the PERIGON pivotal trial, assessing the clinical and ...
Details of early studies using a dual-frequency intravascular ultrasound (IV...
New analysis of the Myval transcatheter aortic valve implantation (TAVI) sys...
FastWave Medical has announced the issuance of its sixth utility patent by t...
The multidisciplinary team at WVU Medicine’s WVU Heart and Vascular Institut...
Medtronic has announced new data for the Evolut transcatheter aortic valve i...
The UK charity for patients with heart valve disease, Heart Valve Voice, has mar...
A Glasgow-based company has announced that three out of four cardiac centres in ...
Abbott has introduced a 35mm sized version of its Navitor Vision transcathet...
Novo Nordisk announced today that Wegovy is now approved to reduce the risk ...
“Disappointed is an understatement,” Michael Reardon (Houston Methodist DeBa...
Abbott has announced the first patient procedures with its investigational t...
One-year data highlighting the performance of the Sapien 3 Ultra Resilia (Ed...
New research will investigate the safety and efficacy of multi-organ, hepati...
The immediate past president of the European Association for Cardio-Thoracic...
EmStop has announced the completion of the CAPTURE-1 early feasibility clinical ...
Supira Medical has announced the initiation and successful patient enrolment in ...
Latest data from the Amulet investigational device exemption (IDE) trial, co...
Ceryx Medical has announced that the first patient has been enrolled in the RSA-...
Medtronic has announced the launch of a randomised study—ALERT—aiming to add...
Second Heart Assist has announced the successful conclusion of its second se...
Three-year results from the OPTION clinical trial, comparing the Watchman FL...
Edoxaban may be an effective alternative to warfarin—the current standard of car...
Routine use of the blood pressure medication spironolactone among patients w...
Hailing from Pamplona, northern Spain, cardiac surgeon Rafael Sádaba was born an...
This advertorial is sponsored by Medtronic
As transcatheter aortic valve ...
In this issue:
Early intervention for aortic stenosis: Trials shed new ligh...
In this issue:
Early intervention for aortic stenosis: Trials shed new ligh...
BrightHeart has received US Food and Drug (FDA) 510(k) clearance for its first a...
Interventional cardiologist Amir Kaki (Heart & Vascular Institute, Detro...
The question of early intervention among patients with severe aortic stenosi...
The routine use of orbital atherectomy prior to percutaneous coronary interv...
Latest data from the ALIGN AR trial, investigating the use of the Trilogy (J...
Late-breaking study results were presented at the TCT 2024 conference as par...
MitrAssist Lifesciences has announced initial clinical results of its Sikelia tr...
Coronary artery bypass graft (CABG) surgery is associated with significantly...
Shockwave Medical, part of Johnson & Johnson MedTech, has announced the ...
Presented today, late-breaking data from the second year of the LIFE-BTK cli...
The US Food and Drug Administration (FDA) has approved Akura Medical’s investiga...
Royal Philips has announced enrolment of the first patient in the US THOR IDE cl...
A retrospective economic analysis of the LIFE-BTK trial has demonstrated the...
The Centers for Medicare and Medicaid Services (CMS) has granted transitional pa...
One-year outcomes of the TRAVEL II study, evaluating the Lux-Valve Plus (Jen...
Six-month data from the PINNACLE I study evaluating the safety and performan...
The Evoque (Edwards Lifesciences) tricuspid valve replacement system demonst...
New analysis of the PROTECTED TAVR trial has shown a reduction in stroke wit...
Medtronic has announced new, long-term data from the SPYRAL HTN-ON MED clini...
Findings from the first international randomised controlled trial (RCT) to c...
Data from the prospective, multicentre, investigator-initiated SIRONA random...
The largest trial to date to study the impact of colchicine—an anti-inflamma...
Vivasure Medical has announced initial positive results from its US IDE PATC...
The Valvosoft non-invasive ultrasound therapy (NIUT) device for the treatment of...
Late-breaking data from the INFINITY SWEDEHEART trial demonstrate significan...
EARLY TAVR lived up to its billing as one of the most hotly anticipated tria...
Shockwave Medical has announced the completion of enrolment in EMPOWER CAD, the ...
Transcatheter aortic valve implantation (TAVI) was no better than a conserva...
Symbiosis, the developer of an adjustable transcatheter mitral valve replace...
Adona Medical has announced the successful first-in-human use of its novel i...
HeartFlow has announced seven-year data confirming the use of its AI-enabled...
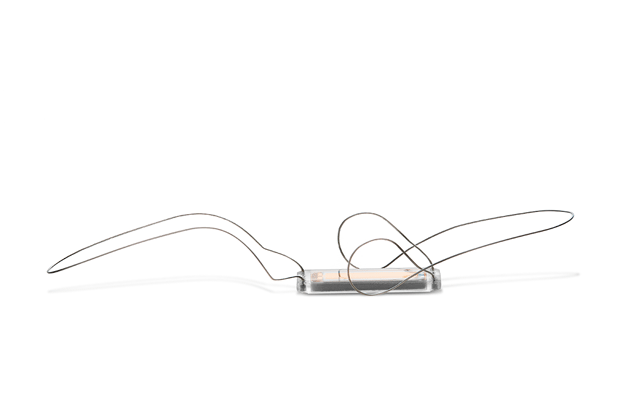
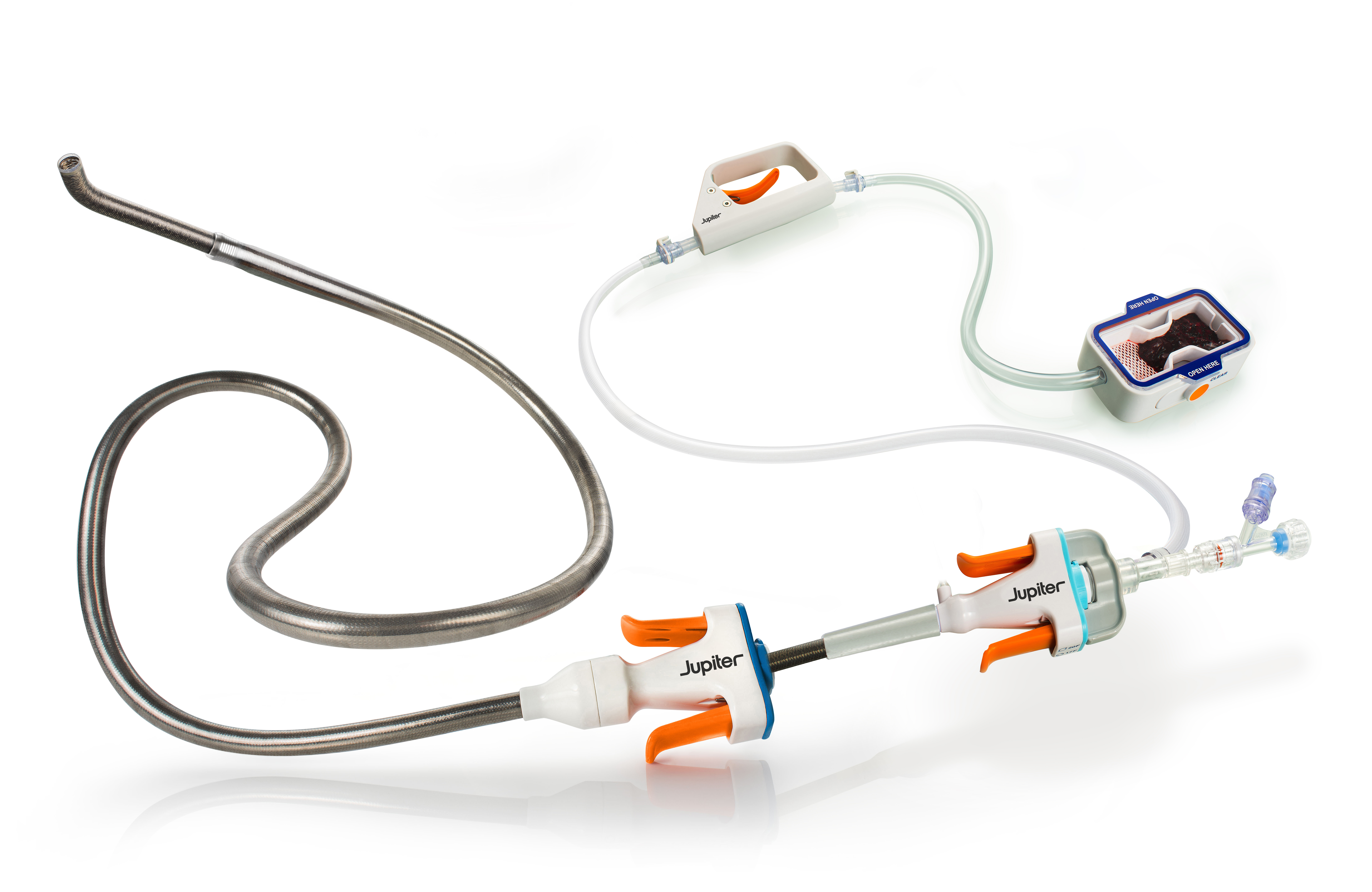
Jupiter Endovascular has announced that the first US patient has been treated in...
Advanced NanoTherapies has announced positive results from its first-in-huma...
Medtronic has announced US Food and Drug Administration (FDA) approval of th...
CroíValve has announced the first patient treated with the Duo system as part of...
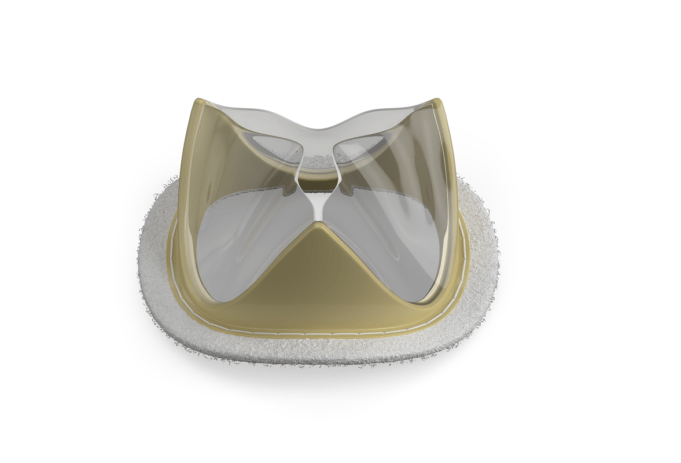
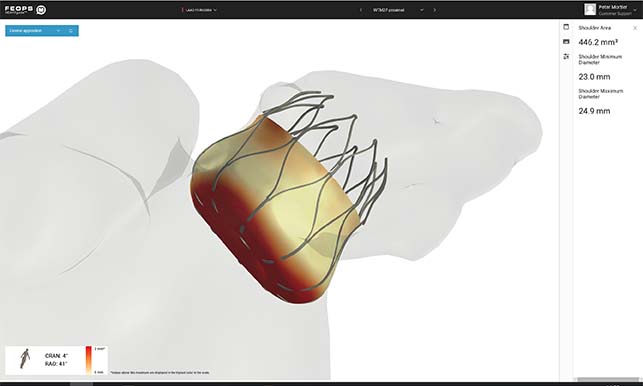
Boston Scientific has announced that the National Institute for Health and Care ...
Medtronic has received CE mark for the Evolut FX+ transcatheter aortic valve...
Tioga Cardiovascular, a Shifamed portfolio company, has announced the first-...
Medtronic has received approval of a US Food and Drug Administration (FDA) inves...
Gentuity has announced that the US Food and Drug Administration (FDA) has grante...
An analysis of data in the UK Biobank has found that COVID-19 infection may incr...
Black patients are 22% more likely than white patients to die in the hospital af...
Robotic aortic valve replacement (RAVR) may offer an alternative to transcat...
Pressing issues in medtech innovation will be on the agenda on the opening d...
Kaminari Medical has closed a €3.8 million funding round with proceeds to be...
Transverse Medical has announced the appointment of Ian T Meredith to the bo...
Patients awaiting cardiac surgery who underwent a digital “prehabilitation” ...
Johnson & Johnson has announced the completion of its acquisition of V-W...
AccurKardia has announced that its aortic valve stenosis electrocardiogram (ECG)...
4C Medical Technologies has commenced enrolment in the ATLAS trial in Europe and...
EMBLOK has announced that it has enrolled the first 50 patients in a clinica...
Reprieve Cardiovascular has announced the completion of enrolment in the FASTR t...
The European Society of Cardiology (ESC) has issued updated guidelines on the ma...
Patients undergoing transcatheter aortic valve implantation (TAVI) do not be...
AtriCure has received regulatory approval to sell the EnCompass clamp in CE-mark...
Conformal Medical has announced the initiation of the GLACE Study, assessing...
Elucid has received 510(k) clearance from the US Food and Drug Administration (F...
This advertorial is sponsored by iVascular
In certain complex percutaneous co...
Cytokinetics has announced that additional analyses synthesizing data from SEQUO...
Endotronix has announced the one-year clinical results from PROACTIVE-HF, ev...
Gore has announced the release of three-year data from the ASSURED clinical ...
Latest guidelines from the European Society of Cardiology (ESC) on the manag...
The US Food and Drug Administration (FDA) has provided market clearance for ...
GE HealthCare has announced that the US Food and Drug Administration (FDA) has g...
Medtronic has announced the launch of a new extracorporeal membrane oxygenat...
De-escalation to ticagrelor monotherapy does not increase ischaemic risk and...
Concerted efforts are needed to address unmet needs and ensure parity of car...
Zoll has announced that the first patient has been enrolled in the SSCORE regist...
A pooled analysis of three large trials failed to demonstrate significant reduct...
Data continue to emerge supporting the safety and efficacy of transcatheter-...
Johnson & Johnson (J&J) recently shared that its medical technology busi...
TRiCares SAS has announced the first implantation of its Topaz transfemoral tric...
Investigators of a trial comparing transcatheter edge-to-edge repair (TEER) ...
Jupiter Endovascular has announced that the first two patients have been tre...
Dasi Simulations has announced that its second product—Dasi Dimensions—has earne...
PlaqueTec and RxCelerate have announced the successful completion of a collabora...
Novostia has announced a leadership transition alongside the successful completi...
Royal Philips today announced the introduction of the 160cm US Food and Drug...
A multicentre, registry-based, randomised trial of the ‘no-touch’ technique ...
Insights from the TESLA registry, an investigator-initiated real-world study o...
SpectraWAVE has announced a US$50M series B funding round which the company ...
This advertorial is sponsored by B Braun Melsungen AG
Diabetic patients w...
CorFlow Therapeutics AG (CorFlow) announced today that it has raised €44 mil...
Brightflow SAS has announces the successful completion of an oversubscribed ...
AISAP, a medical technology company developing AI-powered point-of-care assi...
Venus Medtech has announced that its next-generation balloon-expandable dry-...
The DynamX (Elixir Medical) bioadaptor system met non-inferiority at one year fo...
A randomised trial testing whether the removal of fasting requirements prior to ...
A trial comparing an invasive and a conservative strategy to treat patients over...
The first trial comparing transcatheter aortic valve implantation (TAVI) to surg...
Supplementing potassium for patients who have undergone coronary artery bypa...
A strategy of mitral transcatheter edge-to-edge repair (TEER) plus medical thera...
The European Society of Cardiology (ESC) has today published its 2024 guidelines...
ESC guidelines on the management of elevated blood pressure and hypertension inc...
Jonathan Byrne, consultant cardiologist at King's College Hospital (London, ...
Research presented at the 2024 European Society of Cardiology (ESC) congress...
AtriCure has announced that the first patient has been treated with the AtriClip...
Boston Scientific has obtained CE mark for the Acurate Prime aortic valve sy...
Clinical follow-up using virtual voice technology helped identify complicati...
Brightflow SAS has announced the appointment of Sophie Humbert as chief executiv...
AtriCure has received an expanded indication for the AtriClip left atrial append...
AISAP has announced that the US Food and Drug Administration (FDA) has granted 5...
Nano X Imaging has announced that its deep-learning medical imaging analytics su...
In this issue:
Is it time for rethinking major adverse cardiovascular event...
In this issue:
Is it time for rethinking major adverse cardiovascular event...
Abbott has announced that the US Food and Drug Administration (FDA) has appr...
A new research letter underscores the need to improve long-term survival fol...
A new research letter underscores the need to improve long-term survival fol...
In a paper published in the July 2024 issue of the Journal of Hypertension, Andr...
A meeting centred around live case transmissions from across the world, CX A...
Best known for her work as the principal investigator in the ORBITA trial—a pl...
Johnson & Johnson has entered into a definitive agreement to acquire V-W...
Amplitude Vascular Systems (AVS), developer of the Pulse intravascular lithotrip...
Jupiter Endovascular has exited stealth mode with a US$21 million round of finan...
Genesis MedTech has sold JC Medical to Edwards Lifesciences, in a deal that incl...
Radiaction Medical has announced the formation of its US medical advisory board....
A survey among female cardiology fellows has shown a higher rate of obstetrical ...
Large language models such as ChatGPT and Google Bard could assist heart tea...
BioCardia has submitted a 510(k) for approval of its patented Morph DNA steerabl...
China’s National Medical Products Administration (NMPA) has granted a Class III ...
CroíValve has closed a US$16 million Series B financing round. Proceeds from the...
Three noted cardiovascular trialists have called for a reappraisal of major ...
Teleflex has announced US Food and Drug Administration (FDA) 510(k) clearance of...
For the estimated one in 10 left handers in the general population many day-to-d...
The European Investment Bank (EIB)—the investment arm of the European Union (EU)...
Haemonetics Corporation has announced CE mark certification and the first commer...
PECA Labs has added Joseph E Bavaria (Jefferson Health and Sidney Kimmel Med...
An observational study of patients with type 2 diabetes treated for coronary...
The Texas Heart Institute and BiVACOR, a clinical-stage medical device compa...
Valcare Medical has announced the expansion of the AMEND TS EU pilot study t...
The first patients in the USA have been treated in Recor Medical’s Global Pa...
SoniVie, which has developed a novel Therapeutic Intra-Vascular Ultrasound Syste...
Abbott is rolling out the latest iteration of its Navitor transcatheter aort...
Edwards Lifesciences has announced further expansion of its structural heart por...
Prevencio has been issued a patent from the United States Patent and Trademark O...
TRiCares SAS has announced it has raised US$50 million (€46 million) in a Se...
Magenta Medical, developer of the Elevate heart pump, has closed a US$105 mi...
AtriCure has received regulatory approval from the National Medical Products Adm...
The UK Medicines and Healthcare products Regulatory Agency (MHRA) has approved C...
Royal Brompton Hospital, part of Guy’s and St Thomas’ NHS Foundation Trust (...
Restore Medical has been granted breakthrough device designation by the US Food ...
Elucid has initiated site recruitment for its PRE-VUE CT registry study.
The ...
Materialise, a provider of 3D printing software and services, has acquired F...
iVascular has announced the publication of results from a prospective single...
Monitoring of heart rate and physical activity using consumer wearable devices w...
Early exposure to the use of drug-coated balloons (DCBs) for percutaneous co...
A patient in Cincinnati has become the first in the USA to receive a system ...
Edwards Lifesciences has announced it has exercised its option to acquire Innova...
Ole De Backer (Copenhagen, Denmark) unpacks the results of the NOTION-2 study,...
Edwards Lifesciences has announced Health Canada’s approval of the company's Pas...
Entries have opened for the second edition of the Global Cardiovascular Awards, ...
TruLeaf Medical has received Helsinki Ethics Committee approval in Uzbekista...
Women over the age of 65 who require coronary artery bypass graft (CABG) sur...
Pulsed field ablation (PFA) is safe for treating patients with common types ...
Adona Medical has secured US$33.5 million in Series C financing, with the mo...
Cordis recently announced that the US Food and Drug Administration (FDA) has gra...
At this year’s EuroPCR course (14–17 May, Paris, France), Shifamed presiden...
StarTric has finalised a new seed financing round to advance its next-generation...
Investment in a programme of clinical trials will underpin Boston Scientific’s s...
Biotronik has announced the availability of an expanded maximum allowed diameter...
Anteris Technologies and v2vmedtech, a structural heart company developing a nex...
Reflow Medical has announced the first patient enrolments in DEEPER CORONARY, a ...
Pulnovo Medical Limited has announced the successful initiation and first two-pa...
Valcare Medical has announced the formation of a scientific advisory board (...
MicroPort CardioFlow Medtech Corporation has announced that its self-developed s...
Pie Medical Imaging has announced the completion of enrolment in FASTIII, a mult...
Elixir Medical has announced that its novel bioadaptive implant, the DynamX ...
Cordis has announced positive 24-month results from the Selution SFA Japan trial...
Cardiac magnetic resonance imaging (MRI) data from the first-in-human study ...
Six-month results from the TANDEM I first-in-human clinical trial, investiga...
DeepQure, a Seoul based medical device company with a novel, extravascular s...
Thirty-day results from the Indian clinical study of the Foldax Tria surgica...
Abbott has received CE mark in Europe for the Aveir dual chamber (DR) leadless p...
Initial experience with the Siegel (Mirus) 8Fr transcatheter aortic valve im...
Analysis of data from the TVT registry, looking at the impact of cerebral em...
Results of an analysis of data from the PARTNER trials examining outcomes of...
Edwards Lifesciences has announced that it has entered into a definitive agreeme...
HighLife SAS has announced that the US Food and Drug Administration (FDA) has gr...
Half Moon Medical has announced the treatment of 15 patients with its second...
Johnson & Johnson today announced it has completed its acquisition of Shockw...
InnovHeart has announced the first transseptal clinical trial implant with the p...
Terumo Cardiovascular has announced that the US Food and Drug Administration...
FastWave Medical has announced the 30-day results of its first-in-human (FIH...
Valentin Fuster, president of Mount Sinai Heart and physician-in-chief of th...
Roche has announced that the Tina-quant lipoprotein Lp(a) RxDx assay has receive...
New two-year follow-up data from the BIOMAG-I first-in-human trial confirms ...
Twelve-month outcomes from the DESyne BDS Plus trial, evaluating a novel tri...
Valcare Medical has announced the successful completion of the first two enrolme...
Insights from the investigational device exemption (IDE) pivotal study of the Sh...
GE HealthCare and Medis Medical Imaging have announced a collaboration that ...
Two-year results from the BIOADAPTOR 1:1 randomised controlled trial (RCT), ...
Anteris Technologies has announced the successful completion of a case involving...
Data from the SMART trial—in which it was shown that a self-expanding transc...
Early outcomes following the use of a novel balloon-expandable transcatheter...
Analysis of one-year results from the NOTION-2 trial, in which investigators...
CathWorks has announced that the CathWorks FFRangio system has been approved...
Biotronik has announced the enrolment of the first patient in the BIOMAG-II tria...
Global Heart Hub, an international alliance of heart patient organisations, has ...
In patients with acute coronary syndrome (ACS), intravascular ultrasound (IV...
New data from the REAL-PE analysis investigated catheter-based pulmonary emb...
The advance of transcatheter tricuspid replacement and repair technologies f...
Edwards Lifesciences has announced the European launch of the Sapien 3 Ultra...
4C Medical Technologies has been granted breakthrough device designation by ...
This advertorial is sponsored by GE HealthCare
Experiencing symptoms like che...
Corvia Medical has announced the publication of two-year echocardiographic d...
Findings from the CALORI cardiac catheterisation trial, presented at the Society...
This advertorial is sponsored by Medtronic
Transcatheter aortic valve imp...
New data demonstrate the superiority of radial arterial access compared to f...
Medicine and indeed interventional cardiology were not initially on the rada...
In this issue:
Late-breaking studies from the American College of Cardiolog...
In this issue:
Late-breaking studies from the American College of Cardiolog...
Additional analysis from the SMART trial has demonstrated clinical non-infer...
Medtronic has announced it received approval from the National Medical Produ...
The European Association for Cardio-Thoracic Surgery (EACTS) has launched a ...
Percutaneous coronary intervention (PCI) can be safely performed before, during,...
Results of the early feasibility study evaluating outcomes of the AltaValve ...
Insights from the first-in-human study of the DurAVR (Anteris Technologies) ...
Teleflex has announced that the Wattson temporary pacing guidewire limited marke...
Abbott has announced that the US Food and Drug Administration (FDA) has approved...
The Society of Thoracic Surgeons (STS) has launched new risk calculators coverin...
Medtronic has announced the launch of the Avalus Ultra surgical aortic tissue va...
The American Association for Thoracic Surgery (AATS) and the Cardiovascular Rese...
Biotronik has announced a multinational distribution partnership with Texray...
Concept Medical has announced the commencement of its investigational device exe...
“Unfortunately, we are doing worse for our patients today,” were the soberin...
Foldax has announced that its Tria valves have surpassed 200 patient life ye...
FastWave Medical has announced the issuance of its fourth utility patent by the ...
Simpson Interventions has announced that its Acolyte image-guided crossing and r...
Caranx Medical has announced the successful completion of a robotic transcathete...
Cardiawave SA has appointed of Olivier Pierron as its new chief executive office...
Vivasure Medical has announced the treatment of the first large bore venous pati...
Transverse Medical, developer of the Point-Guard cerebral embolic protection dev...
Acarix has announced the initiation of a US-based clinical study to collect ...
Use of the Impella CP (Abiomed) micro-axial flow pump in the treatment of pa...
People with chronic chest pain who received a coronary sinus reducer (CSR, S...
Medtronic has unveiled top line findings of a survey on women's perceptions ...
Results of the SMART trial, comparing the use of two widely deployed transca...
Patients with heart failure who had an interatrial shunt inserted between th...
Johnson & Johnson is to acquire Shockwave Medical, it has been announced tod...
Automated external defibrillators (AEDs) were used in in 13 of nearly 1,800 ...
Around 10% of all deaths following percutaneous coronary intervention (PCI) are ...
GE HealthCare has announced the launch of Caption AI artificial intelligence...
TRiCares has announced the appointment of Ahmed Elmouelhi as president &...
Michael Reardon and Lars Søndergaard pay tribute to the life and legacy of A...
Debates will shed new light on controversies in the treatment of aortic dise...
Abbott has announced that the US Food and Drug Administration (FDA) has approved...
Protembis has announced the enrolment of the first patient in the PROTEMBO inves...
Merck has announced the US Food and Drug Administration (FDA) has approved sotat...
egnite, a digital health company specialising in cardiovascular care, has announ...
Medtronic has announced that the US Food and Drug Administration (FDA) has a...
HeartFlow has announced the launch of the DECIDE registry, the largest prospecti...
Abbott has announced that the first patient has been enrolled in the ENVISION in...
The use of a radiation shield system—Protego (Image Diagnostics)—could marke...
The first US Food and Drug Administration (FDA) approval of a drug-coated ba...
A company specialising in less invasive structural heart procedures has said tha...
BioCardia and StemCardia has a long-term partnership to advance StemCardia’s inv...
Medtronic has received a license from Health Canada for its Symplicity Spyral mu...
ConKay Medical Systems, a medtech start-up company developing a device for treat...
Best Cardiovascular Product Launch– Siemens Healthineers: ARTIS Family
...
A founding father of transcatheter aortic valve implantation (TAVI), profess...
Medtronic has announced two late-breaking data presentations on four-year ou...
Cardiothoracic surgeon Gilbert Tang (Mount Sinai Health System, New York, US...
This educational supplement, sponsored by Medtronic, explores the latest thinkin...
This advertorial is sponsored by JenaValve
Transcatheter aortic valve imp...
HeartFlow has announced that 10-year follow-up data from its DISCOVER-FLOW s...
SS Innovations International has announced that surgeons have performed the worl...
Saranas has announced results of SAFE-MCS, a multicentre clinical study evaluati...
Results from two large, real-world studies assessing outcomes for patients u...
The US Food and Drug Administration (FDA) has approved an additional indicat...
PulseCath has announced the transition from CE marking to EU Medical Device Regu...
Cleerly has been granted breakthrough device designation by the US Food and Drug...
Six-month results from the PROACTIVE-HF pivotal trial, evaluating the Cordel...
BioCardia has announced interim results from the phase III randomised controlled...
FastWave Medical has announced the issuance of its third utility patent by the U...
Hawthorne Effect, a specialist in technology-enabled mobile clinical researc...
Boston Scientific has announced that it has received US Food and Drug Administra...
New guidelines from the European Association for Cardio-Thoracic Surgery (EA...
Royal Philips has announced the launch of their image-guided therapy mobile ...
FIRE1 today announced that it has completed patient enrolment in the US early fe...
VahatiCor has announced the treatment of the first patient with the A-Flux R...
Like London buses, interventionalists have had a long wait for the US Food a...
Bernardo Cortese (Fondazione Ricerca e Innovazione Cardiovascolare, Lodi, Italy ...
Attaulah Khan Niazi (King Edward Medical University, Lahore, Pakistan) documents...
Foldax has announced a manufacturing agreement with Dolphin Life Science Ind...
Biosense Webster has announced the commencement of patient cases with the invest...
Ismail El-Hamamsy, director of aortic surgery for the Mount Sinai Health Sys...
Laplace Interventional has announced the successful completion of its first-...
Philips' LumiGuide ‘human GPS’ technology is now available to specialised ho...
In this issue:
New data point to shift towards TAVI in patients aged under ...
In this issue:
New data point to shift towards TAVI in patients aged under ...
NeoChord has appointed Todd Berg as chief executive officer to oversee clinical...
For Ajay Kirtane (Columbia University Irving Medical Center/NewYork-Presbyterian...
Two meta-analyses comparing the use of intravascular imaging to angiography ...
BiVACOR, a clinical-stage medical device company, has announced that US$13 milli...
Reprieve Cardiovascular, a development stage company focused on pioneering an au...
Transcatheter aortic valve implantation (TAVI) procedures were more acutely ...
Alain Cribier, the pioneering cardiologist who performed the world’s first t...
The Circulatory System Devices Panel of the Medical Devices Advisory Committee f...
Biotronik has announced the CE approval and launch of the Freesolve resorbable m...
Investigators behind the largest randomised trial to date to assess the use ...
AorticLab has announced CE mark certification for the FLOWer transcatether embol...
Biotronik has launched the Micro Rx novel rapid exchange microcatheter to enhanc...
The REDUCED-1 study, a US Food and Drug Administration (FDA) investigational dev...
Venus Medtech has announced that the VenusP-Valve transcatheter pulmonic valve r...
Genesis MedTech has announced the completion of enrolment in its US early feasib...
A study of more than one million patients undergoing isolated coronary arter...
Edwards Lifesciences has announced that its Evoque tricuspid valve replaceme...
Data from the first US comprehensive single-centre experience at St Bernard’s Me...
Two specialist heart teams are vying to win the Heart Team of the Year Award at ...
A newly published Society for Cardiovascular Angiography and Interventions (...
Royal Philips has received US Food and Drug Administration (FDA) 510(k) clearanc...
Endospan shared 30-day results from the first 22 patients enrolled in the TR...
Jennifer C Romano has been named president of the Society of Thoracic Surgeo...
Research presented at the 60th annual meeting of the Society of Thoracic Sur...
The results of a propensity-matched analysis of all patients undergoing mitral v...
CroíValve has announced the initiation of its early feasibility study (EFS) for ...
Medtronic recently announced that Simona Zannetti has been appointed as the ...
PlaqueTec, a company identifying endotype-specific biomarkers to advance precisi...
Anteris Technologies has announced the successful completion of three new valve-...
Pi-Cardia has received breakthrough device designation from the US Food and Drug...
The full shortlist for the inaugural Global Cardiovascular Awards—which will rec...
Novostia has announced the successful first-in-human implantation of its Triflo ...
An analysis of data from the SPYRAL HTN-ON MED trial of the Symplicity Spyra...
FastWave Medical has announced the successful completion of enrolment for it...
Allowing patients to eat before elective cardiac catheterisation posed no safety...
Cardiac Dimensions, a developer of invasive treatment modalities to address ...
Penumbra has secured CE mark for its Indigo system CAT RX designed to address th...
Occlutech has announced that the US Food and Drug Administration (FDA) has appro...
Realize Medical has announced that its virtual reality (VR) software for surgica...
Orchestra BioMed Holdings has announced the first patient was randomised in the ...
Endovascular Engineering (E2) has announced US Food and Drug Administration (FDA...
People taking medical cannabis for chronic pain have a slightly increased ri...
Cook Medical has announced that its Slip-Cath Beacon Tip hydrophilic selecti...
Proceedings from an expert consensus roundtable that discussed the benefits ...
GE HealthCare has entered into an agreement to acquire MIM Software, a provider ...
Robocath has announced the granting of a marketing authorisation to distribute i...
Endotronix has submitted a premarket approval (PMA) application for its Cord...
Eisenhower Health (Rancho Mirage, USA) is the first hospital in the USA to b...
TRiCares SAS has announced the successful implantation of its Topaz transfemoral...
Laguna Tech USA has announced that a record number five patients were successful...
HeartFlow, a developer of non-invasive artificial intelligence (AI) precisio...
Pulse Biosciences has filed a premarket notification 510(k) to the US Food and D...
Norway-based CardioMech AS, a medical device company developing a transfemor...
Gore has announced the first patient implantation of the Gore ascending stent gr...
Which stories, features and interviews captured the attention of the cardiovascu...
CardioRenal and CHU Grenoble Alpes have announced an innovation that allows chro...
LUMA Vision, developer of a novel four-dimensional (4D) cardiac imaging and ...
Thubrikar Aortic Valve has received authorisation from the Competent Authority o...
Microbot Medical, developer of the Liberty endovascular robotic surgical system,...
Results of the National Cardiogenic Shock Initiative—a single-arm multicentre st...
iVascular has announced the initiation of its first randomised trial of an abbre...
Robocath has announced a first-in-human robotic coronary angioplasty performed a...
Vivasure Medical has announced the first patient treated with PerQseal Elite, th...
Haemonetics Corporation has completed its acquisition of OpSens, the company ann...
A public-private partnership, involving input from clinical and industry partner...
Cardiovascular diseases, including ischaemic heart disease, stroke, heart failur...
Pi-Cardia has appointed Gary Gratson to lead commercial operations for Short...
Patients who received warfarin after bioprosthetic aortic valve replacement had ...
The cardiologist and researcher, Harlan M Krumholz (Yale School of Medicine,...
Details of the first pivotal trial assessing the use of a dedicated leaflet ...
Healthcare 21 (HC21) has gained national reimbursement for Catheter Precision’s ...
Kaneka Corporation has acquired all shares of Japan Medical Device Technology Co...
Investigators behind the BIOPATTERN trial hope that their work will shape th...
Carmat has opened a second production facility in Bois-d'Arcy.
Works on the s...
Medtronic has announced the launch of its Penditure left atrial appendage (L...
Procyrion has announced the enrolment of the first patients in the company’s...
Johnson & Johnson MedTech has announced the completion of the acquisition of...
ReValve Solutions has announced the successful first-in-human use of its Palmett...
The US Food and Drug Administration (FDA) has given the green light for commence...
PlaqueTec has received approval from UK’s Medicines and Healthcare products ...
Inari Medical has announced the first patient enrolment in PEERLESS II. This p...
Foldax has announced completion of enrolment in the Indian clinical trial of...
Abiomed has announced that the first patient in the world has been enrolled in t...
The occurrence of atrial fibrillation (AF) after mitral valve surgery may be mor...
A historic underrepresentation of women in clinical trials for coronary arte...
“It has been a good day for TAVI ,” commented Raj Makkar (Cedars-Sinai, Los ...
Death rates after major surgery are similar regardless of whether a male or fema...
A comparison of two latest-generation self-expanding and balloon-expandable ...
A novel risk score derived from artificial intelligence (AI) may be more eff...
The approval of two renal denervation devices by the US Food and Drug Administra...
Supira Medical announced today that it has received US Food and Drug Adminis...
Global Heart Hub, an international alliance of heart patient organisations, has ...
Medtronic has announced approval from the US Food and Drug Administration (FDA) ...
JenaValve Technology has announced that it will join the American Society of Ech...
This advertorial is sponsored by Medtronic
With transcatheter aortic valv...
Inspired by a passion for nature, Jolanda Kluin began her career in medicine in ...
In this issue:
Low-risk TAVI trials spark optimism, but surgical societies ...
In this issue:
Low-risk TAVI trials spark optimism, but surgical societies ...
Abbott has announced new late-breaking data that show advanced heart failure...
Teleflex has announced the first patient enrolment in a clinical registry that i...
Cleerly has announced the TRANSFORM trial a randomised trial that aims to en...
New analyses from the VOYAGER PAD clinical trial in both high-risk and fragi...
Filterlex Medical has announced the publication of a first-in-human (FIH) st...
Enhanced external counterpulsation (EECP, Flow Therapy), a non-invasive trea...
Vivasure Medical has enrolled the 100th patient in its PATCH clinical study,...
Results from first-in-human (FIH) clinical studies on 40 patients suffering from...
CoreMedic has announced the first successful application of its ChordArt transca...
RCE Technologies, the developer of a non-invasive, instant measurement of ca...
Results of the SELECT clinical trial, presented at the American Heart Associatio...
Xenter unveiled its dual sensor investigational guidewire for transcatheter aort...
Percutaneous coronary intervention (PCI) relieves stable chest pain and impr...
A new study published in BJS Open has found that revascularisation treatment...
The aortic programme at the 2024 Charing Cross (CX) Symposium (23–25 April, Lond...
Medinbox has launched its latest product suites to enhance live collaboratio...
Cordis has announced that its board of directors has appointed Scott Drake a...
Recor Medical has become the first company in the USA to have a device-based...
Conformal Medical has announced positive one-year results from the company's...
Patients with atrial fibrillation (AF) undergoing a transcatheter aortic val...
Data from the REAL-PE study, presented at TCT 2023 (23–26 October, San Franc...
Penumbra, a global healthcare company focused on innovative therapies, annou...
Surmodics has announced the commercial launch of its most advanced hydrophilic m...
Endovascular Engineering (E2) has announced positive initial results from its EN...
Cordis has announced enrolment of the first patient in its SELUTION4DeNovo IDE t...
New research from the FLASH registry shines a light on the effectiveness of ...
Positive results from the ALIGN AR pivotal trial, investigating the use of t...
Laguna Tech USA has announced that the first-in-human clinical case was complete...
Edwards Lifesciences has received CE mark for the Mitris Resilia tissue valve re...
Analysis of health status and quality of life data from the TRILUMINATE Pivo...
A randomised trial comparing transcatheter tricuspid valve replacement (TTVR...
The first US randomised trial investigating the safety and efficacy on the u...
Five-year data from the PARTNER 3 trial, which randomised low surgical risk ...
Results of the LIFE-BTK randomised controlled trial have just been presented...
HeartPoint Global was named winner in the Shark Tank Innovation Competition at t...
Medinol today announced US Food and Drug Administration (FDA) approval for t...
At four years of follow-up, patients with severe aortic stenosis at low risk...
Data from a patient-level meta-analysis—a factor in the US Food and Drug Adm...
GE HealthCare has announced US Food and Drug Administration (FDA) 510(k) clearan...
Patients have passed through the multi-month enhanced screening process and ...
New research examining the safety and efficacy of using radial access for pe...
Researchers behind a trial to quantify the true prevalence of valvular heart dis...
Medtronic has received CE mark for the Evolut FX transcatheter aortic valve impl...
A population-based cohort study of patients treated in the Austrian healthca...
OpSens has entered into a definitive arrangement agreement with Haemonetics Corp...
FEops has entered into a partnership with ConcertAI’s TeraRecon for the comm...
Endotronix has received investigational device exemption (IDE) approval from...
Roger Malcolm Greenhalgh, the surgeon internationally renowned for his unpar...
Data from the On-X aortic heart valve low INR post-market study, presented d...
Elixir Medical has announced submission to Japan’s Pharmaceutical and Medica...
Artivion has announced presentation of interim results from the Ascyrus medical ...
Ongoing statin treatment may be beneficial for patients after isolated surgi...
A new study has shed fresh light on the potential connection between pre-existin...
Anumana has announced US Food and Drug Administration (FDA) 510(k) clearance for...
MedAlliance has been acquired by Cordis for a 2022 investment of US$35 million a...
EnCompass Technologies has announced that the US Food and Drug Administration (F...
Boston Scientific has announced US Food Administration (FDA) clearance of the AV...
Surgeons at the University of Maryland Medical Center (UMMC, Baltimore, USA) hav...
Inorganic nitrate reduces contrast-induced nephropathy (CIN), improves renal out...
Shockwave Medical has announced that Nick West has joined the company in the...
As one of the UK’s leaders in interventional cardiology, David Hildick-Smith (Un...
In this issue:
Societies weigh in on renal denervation as US Food and Drug ...
In this issue:
Societies weigh in on renal denervation as US Food and Drug ...
Pi-Cardia has announced the successful completion of its ShortCut pivotal study ...
This advertorial is sponsored by Medtronic
Hypertension is a leading cause of...
A survey of cardiac surgeons and interventional cardiologists from 26 European n...
SpectraWAVE has announced US Food and Drug Administration (FDA) 510(k) clearance...
Saranas has completed enrolment in SAFE-MCS, a multicentre, single arm, open-lab...
MedAlliance has announced enrolment of over 1,660 patients in its SELUTION DeNov...
FastWave Medical recently announced the swift closure of an oversubscribed...
HighLife SAS has announced the introduction of a new valve size in its clinical ...
Ten-year data from the NOTION trial—the longest-running clinical trial to da...
Boston Scientific has received US Food and Drug Administration (FDA) approva...
The first patient has been enrolled in a UK study of large vessel de novo corona...
The first-in-man (FIM) use of the NyokAssist (magAssist) percutaneous ventri...
A study assessing the impact of the addition of adding coronary computed tom...
One-year-data from the BIOFLOW-DAPT trial demonstrated non-inferiority and a goo...
Protembis has announced the appointment of J Mocco (Icahn School of Medicine at ...
Radiaction Medical has raised US$12.6 million in Series C2 funding led by US pri...
The UK’s Medicines and Healthcare products Regulatory Agency (MHRA) has desi...
Extended P2Y12 inhibitor monotherapy beyond 12 months after percutaneous corona...
A joint review of evidence into the treatment of patients at low surgical-risk w...
Early extracorporeal life support (ECLS) does not improve survival in patien...
Results of the first randomised trial comparing pulsed field ablation (PFA) ...
GE Healthcare has announced the launch of two new tools for the cardiology m...
Data from three randomised trials and a meta-analysis presented at the Europ...
The Society for Cardiovascular Angiography & Interventions’ (SCAI’s) po...
Siemens Healthineers has unveiled the Acuson Origin, a dedicated cardiovascular ...
The Society for Cardiovascular Angiography & Interventions (SCAI) has welcom...
A simple blood test may predict the risk of progressive kidney and heart disease...
The US Food and Drug Administration (FDA) Circulatory Systems Devices Panel ...
The heart teams at the Piedmont Heart Institute (Atlanta, USA) and the Unive...
The US Food and Drug Administration (FDA) Circulatory Systems Devices Panel of t...
Myocardial infarction (MI) patients who do not take daily aspirin have an elevat...
Enrolment has been completed in the US Food and Drug Administration (FDA)-approv...
Advanced NanoTherapies—ANT—has announced the completion of a US$4million Ser...
The Society for Cardiovascular Angiography & Interventions (SCAI) has releas...
The British Heart Foundation (BHF) has appointed research leader and NHS con...
Latest research in the field of renal denervation has prompted its inclusion...
AstraZeneca’s Forxiga (dapagliflozin) has been approved in China for use in adul...
Heart failure start-up Acorai has announced the initiation of a global clini...
An artificial intelligence (AI) model may be more efficient at detecting signatu...
Research published in the journal Circulation: Cardiovascular Quality and Ou...
A new digital tool launched by the Society of Thoracic Surgeons (STS) draws ...
Viz.ai has received a de novo approval by the US Food and Drug Administratio...
CytoSorbents has announced the completion of the pivotal STAR-T (Safe and Timely...
Xeltis has announced the closing of an additional €12.5 million in funding from ...
Orchestra BioMed Holdings has been granted US Food and Drug Administration (FDA)...
Anteris Technologies has reported that its DurAVR biomimetic, single-piece t...
Ilika and Cirtec Medical have announced that they have signed a 10-year manufact...
Venus Medtech has announced US Food and Drug Administration (FDA) Investigationa...
Canon Medical has introduced its Alphenix / Evolve Edition angiography system fo...
Ian Meredith is a well-known figure within the field of interventional cardi...
Hitting the headlines in July were new European guidelines on the treatment ...
Shockwave Medical has announced that as part of the Fiscal Year 2024 Medicare Ho...
The World Health Organization (WHO) has included a cardiovascular polypill—an al...
Peijia Medical has announced that the first Chinese patient has been enrolled in...
Laplace Interventional, a medical device company developing a transcatheter ...
Foldax has announced that the first 30 patients have been treated outside of...
Genesis MedTech has announced that its J-Valve transfemoral (TF) system has been...
Applying artificial intelligence (AI) to a single apical four chamber (A4C) ...
Respective results from the REVEALPLAQUE, DECODE, and SMART-CT were presente...
Anteris Technologies has announced that the DurAVR transcatheter heart valve...
RapidAI today announced US$75 million in Series C funding led by Vista Credit Pa...
CardioPrecision, a developer of less invasive treatment solutions for structural...
Innovation is a part of the DNA of cardiothoracic surgery, Friedhelm Beyersd...
HeartBeam, developer of a 3D-vector electrocardiogram (VECG) platform, has a...
Researchers will carry out the first clinical trial focusing on women and mi...
The combination of soaring heat and fine particulate pollution may double the ri...
Entries are now open for the inaugural edition of the Global Cardiovascular Awar...
Onecrea Medical has announced the success of the first-in-human implants of its ...
PECA Labs has added Jeffrey P Carpenter, Jeffrey O’Donnell Sr and Samuel E Navar...
Interventional cardiologists need to have a range of approaches and devices i...
The president of the Society for Cardiovascular Angiography & Interventi...
Avertix Medical—formerly known as Angel Medical Systems—has announced that I...
The TIMELY consortium announced today the enrolment of the first patient in the ...
ReCor Medical and its parent company Otsuka Medical Devices have welcomed th...
Elixir Medical has announced enrolment completion in INFINITY-SWEDEHEART, a ...
A dedicated, prospective randomised controlled trial (RCT) investigating the saf...
Citizens of Europe at risk for heart attacks, stroke and chronic kidney disease ...
Results of a stent-level analysis from the HOST-IDEA study, which compared t...
In a major Swedish study, researchers have discovered a link between the lev...
The release of a letter from the US Food and Drug Administration (FDA) concernin...
Following yesterday's news that the US Food and Drug Administration (FDA) has ch...
Verve Medical has received approval from the US Food and Drug Administration (FD...
FIRE1 has announced that the first US patients have been successfully implanted ...
In a letter to healthcare providers dated 11 July 2023, the US Food and Drug...
Sequana Medical NV has announced that the first patient has been enrolled in the...
Alvimedica has announced the launch of the Alvision Kaplan curves radial portfol...
Physicians at the James Cook University Hospital (Middlesbrough, UK) have pe...
Researchers have developed a soft, fully bioresorbable, transparent microelectro...
Abbott has announced that the US Food and Drug Administration (FDA) has approved...
EBC MAIN investigator David Hildick-Smith (Brighton, UK) delivers key messag...
In the wake of the release of update guidelines from the European Hypertension S...
The combined presence of chronic kidney disease and diabetes among women undergo...
Researchers have found that the minimum level of oxygen delivery during cardiopu...
Translumina has announced the acquisition of Blue Medical Devices, a Wellinq...
CathWorks has announced that the first patient has been enrolled in the Adva...
Patients who feel low when having a cardiac device implanted are more likely...
New and expanded guidelines from the European Society of Hypertension (ESH) conc...
Anumana has received breakthrough device designation from the US Food and Drug A...
Vygon, a specialist in the design, manufacturing and marketing of medical device...
A new study from Keck Medicine at the University of Southern California (USC...
The US Food and Drug Administration (FDA) has approved the use of iTFlow in bloo...
CorWave has raised €61 million to fund its first industrial deployment and entry...
egnite and JenaValve Technology have announced a strategic partnership aimed to ...
Nearly one-third of patients with an implanted device to prevent sudden deat...
PECA Labs has announced the successful first-in-human implant of the MASA valve,...
In order to avoid coronary obstruction—a rare but “nasty” complication of tr...
Teleflex has received US Food and Drug Administration (FDA) clearance for the Wa...
Patients with ST-elevation myocardial infarction (STEMI) occurring during th...
Results of the first study of a first-generation drug-coated balloon (DCB) w...
Dasi Simulations has announced it has received US Food and Drug Administration (...
The European Society of Cardiology (ESC) has announced the appointment of Je...
Translumina has announced the acquisition of Munich-based Lamed GmbH, which ...
The US Food and Drug Administration (FDA) has released draft guidance with u...
While the exact location of atherosclerotic plaque ruptures—a common cause of my...
Defibrillators are being used in just one in 10 cardiac arrests where they are a...
Lars Søndergaard has left his role as professor of cardiology at Rigshospitalet,...
Conformal Medical has announced the successful completion of its series D fu...
EnCompass Technologies has announced its novel F2 cerebral embolic filter was us...
Medtronic has announced that Ken Washington has been appointed chief technology ...
Valve Medical, a wholly owned subsidiary of Medinol Ltd, has announced the first...
Discharging patients early after transcatheter aortic valve implantation (TA...
This advertorial is sponsored by Boston Scientific
Stroke—particularly disabl...
New data presented from an investigator-sponsored European trial found managing ...
Thirona recently announced its new artificial intelligence (AI)-based algorithm ...
Cora Therapeutics has announced the presentation of the results of a clinica...
An international clinical trial presented today at the European Stroke Organ...
Implementation of a dedicated training programme allowed hospitals to incre...
Enrolment in the EURO SHOCK trial was curtailed due to the COVID-19 pandemi...
Ticagrelor monotherapy after three months of ticagrelor plus aspirin was ass...
Preliminary results from the BIO-LIBRA study, assessing the outcomes of devi...
Results from a pivotal clinical trial found a leadless pacemaker can deliver...
Inventor of the Valsalva aortic graft Ruggero De Paulis (European Hospital Unica...
In this issue:
A new era for intracoronary imaging
Top trials from ACC 2...
A large registry study of patients undergoing transcatheter edge-to-edge rep...
A novel artificial intelligence (AI) model correctly identified patients at near...
Analysis of the Global SYMPLICITY registry DEFINE (GSR DEFINE), presented at...
Paolo Contessotto (National University of Ireland Galway, Galway, Ireland) outli...
Bentley has announced the US launch of its BeBack crossing catheter, which is de...
A randomised controlled trial comparing the safety of distal versus proximal rad...
Six-month outcomes from FLASH registry have shown that patients with pulmonary e...
Medtronic has announced two-year results for its Harmony transcatheter pulmonary...
Results of the first-in-human study BIOMAG-I study, assessing Biotronik’s la...
Analysis from The North American COVID-19 STEMI (NACMI) registry, presented ...
The Society for Cardiovascular Angiography & Interventions (SCAI) has na...
Elixir Medical has announced that the BIOADAPTOR RCT trial of the company’s ...
New data from the Benchmark Registry, a study of a streamlined transcatheter aor...
A process of flushing prosthetic valves using carbon dioxide (CO2) prior to ...
First-generation bioresorbable vascular scaffolds (BVS) may be just as effec...
A machine learning-based model which uses electronic health record (EHR) dat...
Edwards Lifesciences has announced new data from the COMMENCE aortic trial, ...
A real-world comparison of two techniques aimed at reducing the risk of coro...
Operators performing percutaneous coronary intervention (PCI) in bifurcation...
The use of an image-guidance system that overlays angiographic images on liv...
In this issue:
A new era for intracoronary imaging
Top trials from ACC 2...
Robocath has announced the launch of its latest robotic platform—the R-One+.
...
Shockwave Medical has announced the full commercial availability of the Shockwav...
Foldax has announced that it has executed an agreement with Australia’s nati...
OpSens has announced the inclusion of SavvyWire in the COMPLETE TAVR clinical st...
In a recent study, researchers determined that derivatives of natural emulsi...
Postmenopausal women with clogged arteries are at higher risk of heart attac...
This educational supplement, sponsored by Shockwave Medical, gains fresh perspec...
How has the new Shockwave C2+ catheter changed the treatment of calcified co...
Calcified nodules are frequently identified among coronary artery lesions an...
Tom Johnson (University Hospitals Bristol & Weston NHS Foundation Trust,...
Akura Medical announced today it has initiated its first-in-human clinical study...
This advertorial is sponsored by Medtronic
New data offer greater perspec...
This advertorial is sponsored by B Braun Melsungen AG
“The evolution of PCI ...
A recent study has found deep neural networks (DDNs)—a category of artificia...
AstraZeneca’s Farxiga (dapagliflozin) has been approved in the USA to reduce the...
Emboline has announced that the first patient has been treated in the Protect th...
Judges of the CX 2023 Dragon’s Den-style contest — the finale of the Day 3 C...
News highlights this April were brought by Podium First data from the BASIL-...
Vivasure Medical has announced it has enrolled the first patients in the com...
The US Food and Drug Administration (FDA) has granted the third investigational ...
Research has highlighted significant regional variations in the availability...
Statin treatment after aortic repair is associated with improved long-term s...
Ultromics has been granted US Food and Drug Administration (FDA) breakthrough de...
A question from Manj Gohel (Cambridge University Hospitals NHS Foundation Trust,...
Cardiac Dimensions has announced that its Carillon mitral contour system, fo...
Cardialysis, the 40-year old cardiovascular research organisation, based in ...
Pie Medical Imaging has received NINSHO Medical Device Certification for CAAS Qa...
Effective management of depression through psychological therapy is associated w...
Adept Medical has launched the Adducted Arm Scoop—an improved solution for s...
The first transcatheter tricuspid valve replacement procedure in the USA usi...
Deaths from cardiovascular disease are elevated on polluted days and for two day...
Shockwave Medical has announced the completion of its previously announced a...
OpSens has announced the successful completion of enrolment in the SAFE–TAVI cli...
The news agenda in March was dominated by late-breaking data from the American C...
AVS, an early-stage medical device company focused on safely and effectively...
New research will investigate how stretching and blood flow can inhibit or e...
Medtronic has announced the launch of MRI Care Pathway, a new system designed to...
Endotronix has announced its PROACTIVE-HF pivotal study has successfully com...
Wearable devices such as smart watches could be used to detect a higher risk of ...
The UK’s first remotely-supported transcatheter aortic valve implantation (TAVI)...
Cardiovascular Systems has announced the complete enrolment of its 2,000-pat...
The Society for Cardiovascular Angiography & Interventions (SCAI) and th...
Abbott has announced that the US Food and Drug Administration (FDA) has appr...
Supira Medical has announced the appointment of Azeem Latib (Montefiore Heal...
This advertorial is sponsored by Boston Scientific
Today there are more than ...
Results from the PADN-5 study, investigating the efficacy and safety of pulm...
Saranas has announced that it has reached the midpoint for enrolment in SAFE-MCS...
A prospective observational study had found that, in asymptomatic persons, s...
Procyrion, a medical device company which aims to improve outcomes...
Abbott has today announced new data that found monitoring patients...
MedAlliance has announced enrolment of over 1,000 patients in its SELUTION DeNov...
Immediate, complete revascularisation with percutaneous coronary interventio...
Stereotaxis has announced the opening of a new robotic electrophysiology pro...
A total of £10 million has been awarded to the Medicines and Healthcare products...
Merit Medical has announced the expansion of itsSwiftNinja steerable microcathet...
Angiographic and clinical data from the BIOMAG-I clinical study, presented a...
HeartBeam, a cardiac technology company that has developed the three-dimensional...
Vivasure Medical has announced that the US Food and Drug Administration (FDA) ha...
LivaNova has announced the receipt of US Food and Drug Administration (FDA) 510(...
Assessing myocardial viability does not aid the selection of patients who wi...
February’s trending topics included a look at the past, present and future of tr...
A new trial of endovascular ultrasound-based renal denervation technology ha...
Edwards Lifesciences has highlighted new data examining mortality rates and ...
The European Union’s Council of Ministers has today adopted a resolution to exte...
Data from a new prespecified analysis of the phase III VOYAGER PAD clinical ...
Results of the Pulsed AF Pivotal trial, investigating pulsed field ablation ...
The largest randomised controlled trial to date to compare minimally invasiv...
Neovasc has announced that its shareholders have approved the previously-ann...
Use of intravascular imaging guidance for percutaneous coronary intervention (PC...
Large bore mechanical thrombectomy with the FlowTriever system (Inari Medica...
Final, five-year clinical outcomes from the COAPT trial, evaluating transcat...
Three-year results from the Evolut Low Risk trial have shown “durable” benef...
Hotly-anticipated results of the randomised TRILUMINATE pivotal trial, prese...
One-year insights from the bRIGHT registry, detailing real-world results of...
Researchers have detailed a new preclinical model for non-ST elevation myocardia...
Women have been found to have significantly higher risk of operative mortali...
SpectraWAVE has announced US Food and Drug Administration (FDA) 510(k) clearance...
Results from the PORTICO NG study of Abbott’s Navitor transcatheter aortic valve...
ReCor Medical and its parent company, Otsuka Medical Devices, recently annou...
Five-year bioprosthetic valve dysfunction data for Medtronic’s CoreValve and...
Results of a trial involving cell therapy in patients with chronic heart fai...
Results from the CONFORMAL early feasibility study (EFS), evaluating the use...
A model designed to predict surgical risk in the setting of adult congenital hea...
Medtronic has welcomed the publication of the consensus statement on renal dener...
Procyrion has announced the successful treatment of the first patients in the A ...
Disabilities were underreported in clinical trial data and commonly used as a ba...
Medtronic has announced the relaunch of its Harmony transcatheter pulmonary valv...
CVRx has announced the preliminary topline results of the BeAT-HF—Baroreflex act...
RapidAI announced it has received US Food and Drug Administration (FDA) 510(k) c...
The publication of a consensus statement from the European Society of Cardio...
Teleflex Incorporated has today announced key milestones in the release of two n...
A single-centre retrospective study of rehospitalisation after transcathete...
Xeltis has raised €32 million in a Series D2 equity fundraise, backed by a syndi...
NOTE: This video is ONLY available to watch in selected countries and geographie...
The American College of Cardiology (ACC), the American Heart Association (AHA) a...
Renal denervation represents another treatment option in patients with uncontrol...
As transcatheter aortic valve implantation (TAVI) moves beyond its 20-year ...
Sleeping an inconsistent number of hours each night and falling asleep at di...
Attendees of the 2023 Joint Interventional Meeting (JIM, 9–11 February, Milan, I...
SIS Medical AG has announced the launch of its OPN NC percutaneous transluminal ...
GE HealthCare has signed an agreement to acquire Caption Health, a developer...
Abbott and Cardiovascular Systems (CSI) have announced a definitive agreement fo...
A retrospective cohort study has found the likelihood of receiving percutane...
In the opening month of 2023, Cardiovascular News’ most read stories include sex...
This advertorial is sponsored by Cardionovum
“Everything started with bal...
A randomised study led by Greater Manchester Mental Health NHS Foundation Tr...
In this issue:
Medicare study reopens guideline debate around surgery and P...
In this issue:
Medicare study reopens guideline debate around surgery and P...
This advertorial is sponsored by iVascular
A new coronary scoring balloon...
Performance improvement company Biome Analytics has announced the publication of...
TruLeaf Medical is to conduct a first-in-human clinical trial of a “two-stag...
Percutaneous coronary intervention (PCI) without on-site surgical support is as ...
From cutting-edge aortic interventions to consensus on revascularisation strateg...
This advertorial is sponsored by B. Braun Melsungen AG
The modern field o...
AtriCure has announced that the first patient was treated in the LeAAPS clinical...
Trials in coronary imaging, transcatheter tricuspid valve interventions, tra...
Little progress has been made over the last decade in improving gender balance i...
Interventional cardiology is a rapidly evolving field, offering the opportunity ...
An analysis of data from more than 100,000 patients with multivessel coronar...
The first US patient has been enrolled at Medstar Washington Hospital Center (Wa...
BioVentrix, a privately held medical device company focused on the development o...
A group of engineers and physicians have created a wearable ultrasonic devic...
Transcatheter treatment options for aortic regurgitation (AR) bring a new, ...
A study conducted by Michigan Medicine (Ann Arbor, USA) researchers suggests tha...
A new risk assessment tool has been produced to provide a comprehensive meth...
Pie Medical Imaging has announced the enrolment of the 500th patient in the FAST...
Zoll has announced that the first patient has been enrolled in the REAL SSO2 pos...
The largest dedicated registry on mitral transcatheter edge-to-edge repair (...
Shockwave Medical has entered into a definitive agreement to acquire Neovasc...
The US Food and Drug Administration (FDA) has approved the Navitor (Abbott) tran...
Research evaluating the effects of high bleeding risk (HBR) and complex percutan...
Pie Medical Imaging has launched a new version of its echocardiography software ...
Viz.ai has announced the launch of Viz Vascular Suite—artificial intelligenc...
The UK’s National Institute for Health and Care Excellence (NICE) has published ...
HVR Cardio has closed on a €10.7 million series B round of financing. The Se...
Patients with depression were 10–20% less likely to adhere to guideline-directed...