Foldax has announced that it has executed an agreement with Australia’s national science agency, CSIRO, for full ownership of a polymer technology patent family.
Foldax has incorporated the polymer technology into its LifePolymer materials platform and has conducted testing, which indicates the materials are compatible with blood, with low adherence of proteins, cells, and calcification. This LifePolymer material characteristic makes it highly biocompatible with the human body and extremely durable, which may be ideal for certain medical devices, the company said in a press release.
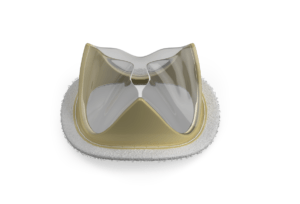
Foldax’s initial development with the patented LifePolymer material is for its portfolio of Tria heart valves, which combines the polymer material with valve leaflet design intended to avoid calcification, withstand stresses and strains without failure, and restore patient quality of life without lifelong use of anticoagulant medication.
The LifePolymer technology has undergone extensive biological safety testing that has been included in Foldax’s regulatory filings to the US Food and Drug Administration (FDA). The Tria biopolymer heart valve is the first polymer valve to receive FDA approval for human studies in both aortic and mitral positions.
“While we believe LifePolymer is ideal for heart valves, we are also excited about the possibility of its application to other medical devices,” said Gregory D Casciaro, Foldax CEO. “The biocompatible and durable material properties of LifePolymer may make it potentially ideal for drug delivery, orthopedics, glucose monitoring, cardiac cannula, blood pumps, blood oxygenation circuits, vascular, stent coverings, electrophysiology, and non- medical coatings.”
The Tria heart valve and LifePolymer technology are considered investigational and are not available for commercial sale in the USA.












