Elixir Medical has announced submission to Japan’s Pharmaceutical and Medical Device Agency (PMDA) for the approval of the DynamX coronary bioadaptor system for the treatment of coronary artery disease (CAD).
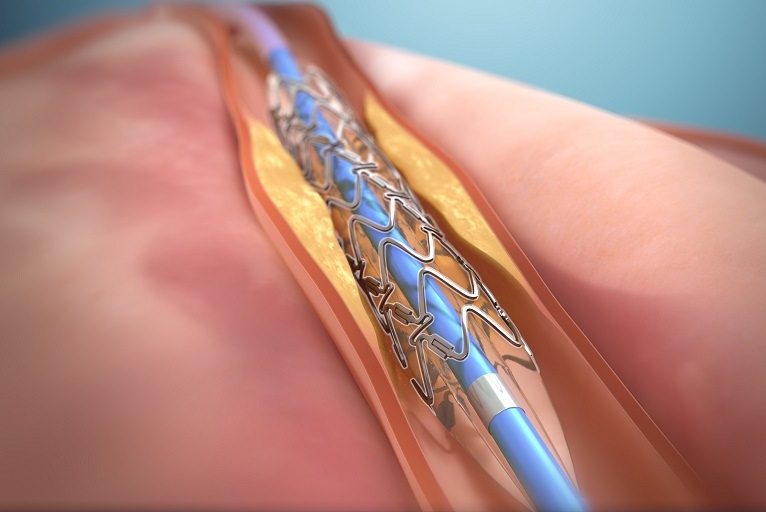
DynamX coronary bioadaptor was developed to overcome the limitations of drug-eluting stents (DES) and bioresorbable scaffolds (BRS). The device is a novel coronary implant designed to unlock the scaffold, uncage the vessel, and provide the essential dynamic support after uncaging to return normal vessel motion and function after percutaneous coronary intervention (PCI).
In a randomised controlled trial versus DES, the unique mechanism of action has shown reduced target lesion failure (TLF) rates and restored vessel pulsatility, translating to increased blood flow, improved vessel lumen diameter, and reduced plaque progression.
“Our submission for approval in Japan is an exciting milestone for the company and industry. The DynamX coronary bioadaptor represents a culmination of major design and manufacturing breakthroughs and three clinical trials consistently demonstrating differentiated functional performance compared to the standard of care,” said Motasim Sirhan, CEO of Elixir Medical. “Coronary artery disease treatment with stents has seen no major innovation in over 20 years, and we are thrilled to challenge the current standard of care by bringing our technology to physicians and patients around the world.”
“Drug-eluting stents have served an important role in the treatment of coronary artery disease, but have yet to overcome many challenges, including restricting vessel motion and function, mechanical failure, and progression of plaque,” said Shigeru Saito (Shonan Kamakura General Hospital, Kamakura, Japan), principal investigator of the BIOADAPTOR RCT trial.
“What we have seen with the DynamX in the 12-month BIOADAPTOR RCT data exceeded our expectations against the current standard of care—the Resolute Onyx DES—in clinical outcomes, and for the first time ever demonstrated restoration of vessel pulsatility, motion and function by uncaging the vessel while providing the needed support after uncaging. The findings collectively point to a technology standard not seen before that I believe is of great benefit to patients.”