
Calcified nodules are frequently identified among coronary artery lesions and are associated with poor percutaneous coronary intervention (PCI) results and adverse long-term results. The best strategy to adequately manage this situation remains debated, says Nicolas Amabile (Institut Mutualiste Montsouris, Paris, France), who reports a case in which intravascular lithotripsy (IVL) was successfully used to treat calcified nodules in a challenging location.
Case presentation
A 60-year-old man was referred to our cath lab for a coronary angiography. His medical history was significant for stable multivessel coronary artery disease: he benefited from coronary artery bypass grafting (CABG) (left internal mammary artery [LIMA] left anterior descending [LAD] & right obtuse mammary artery [RIMA] to obtuse marginal artery [OM1]) ten years prior, and subsequent ramus and circumflex PCIs three years before his admission. The patient had been suffering from recurrent angina for the previous two months and a stress echocardiography revealed ischaemia in the inferior left ventricular (LV) walls (three segments).
Coronary angiography depicted occluded LAD, occluded circumflex and patent ramus stents. Moreover, LIMA and RIMA grafts presented no abnormalities and downstream native vessels were correctly visualised. In addition, the ostial RCA presented a tight, calcified, severe stenosis creating pressure-damping when cannulated (Figure 1). Severe aortic wall calcifications were also observed. This angiographic aspect was compatible with a de novo calcified nodule.
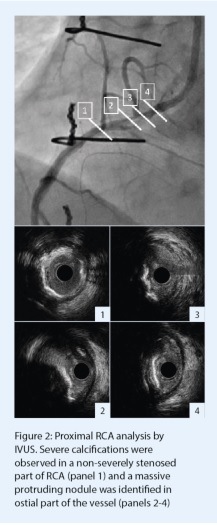
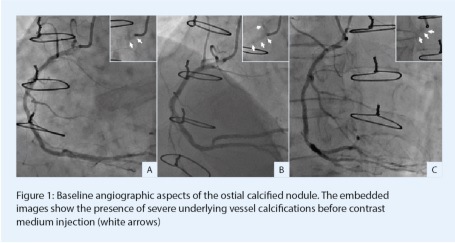 In order to better characterise the right coronary RCA lesion and prepare further plaque modification before stent implantation, an HD-intravascular ultrasound (IVUS) analysis (OptiCross catheter, Boston Scientific) was performed. This intracoronary imaging modality was chosen because of the excellent catheter profile and its ability to accurately assess coronary ostia morphology and dimensions. The initial IVUS run showed diffuse and severe calcifications on proximal RCA with some concentric and eccentric moderate stenoses (Figure 2,1). A massive eruptive calcified nodule, defined as a convex shape of luminal surface and luminal side of calcium with protrusion into the coronary artery lumen,1 was observed at the junction between the aorta and RCA (Figure 2, 2-4). The minimal residual luminal area was measured to 2.8mm2 at this site.
In order to better characterise the right coronary RCA lesion and prepare further plaque modification before stent implantation, an HD-intravascular ultrasound (IVUS) analysis (OptiCross catheter, Boston Scientific) was performed. This intracoronary imaging modality was chosen because of the excellent catheter profile and its ability to accurately assess coronary ostia morphology and dimensions. The initial IVUS run showed diffuse and severe calcifications on proximal RCA with some concentric and eccentric moderate stenoses (Figure 2,1). A massive eruptive calcified nodule, defined as a convex shape of luminal surface and luminal side of calcium with protrusion into the coronary artery lumen,1 was observed at the junction between the aorta and RCA (Figure 2, 2-4). The minimal residual luminal area was measured to 2.8mm2 at this site.
 PCI was decided on the ostial and proximal RCA. According to the lesion characteristics, IVL was proposed. The stenosis was predilated with a 3x12mm non-compliant balloon that was inflated up to 12atm for 20 seconds. A Shockwave C2+ IVL catheter was subsequently advanced into the target vessel and 12 cycles of 10 pulses were delivered in the proximal and ostial RCA, leading to the progressive disappearance of the residual imprint on the balloon (Figure 3, A-C).
PCI was decided on the ostial and proximal RCA. According to the lesion characteristics, IVL was proposed. The stenosis was predilated with a 3x12mm non-compliant balloon that was inflated up to 12atm for 20 seconds. A Shockwave C2+ IVL catheter was subsequently advanced into the target vessel and 12 cycles of 10 pulses were delivered in the proximal and ostial RCA, leading to the progressive disappearance of the residual imprint on the balloon (Figure 3, A-C).
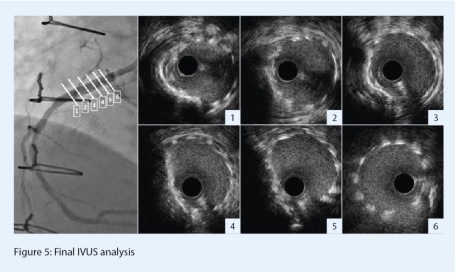
 Post-IVL angiography showed a significantly improved angio aspect with mild residual stenosis (Figure 3, D). A 3.5x24mm everolimus-eluting stent (Synergy Megatron, Boston Scientific) was then implanted. The device diameter and length were determined according to the dimensions of the distal landing zone by IVUS. In order to prevent any geographical miss, a mild device protrusion in the aorta was applied (Figure 4, A-B). Finally, additional post-dilation was performed with a 3.5x12mm non-compliant balloon that was inflated up to 14atm (Figure 4, C). Final angio results depicted no residual stenosis (Figure 4, D). Post-PCI IVUS analysis confirmed adequate results: there was no edge dissection, nor strut significant malapposition. The device section was mostly symmetric and circular, suggesting that the initial calcium nodule did not affect stent expansion after the lesion was prepared by IVL (Figure 5). The minimal stent area was measured as 7.9mm2, which represented a significant increase compared to the baseline minimal lumen area. The subsequent clinical evolution was uneventful.
Post-IVL angiography showed a significantly improved angio aspect with mild residual stenosis (Figure 3, D). A 3.5x24mm everolimus-eluting stent (Synergy Megatron, Boston Scientific) was then implanted. The device diameter and length were determined according to the dimensions of the distal landing zone by IVUS. In order to prevent any geographical miss, a mild device protrusion in the aorta was applied (Figure 4, A-B). Finally, additional post-dilation was performed with a 3.5x12mm non-compliant balloon that was inflated up to 14atm (Figure 4, C). Final angio results depicted no residual stenosis (Figure 4, D). Post-PCI IVUS analysis confirmed adequate results: there was no edge dissection, nor strut significant malapposition. The device section was mostly symmetric and circular, suggesting that the initial calcium nodule did not affect stent expansion after the lesion was prepared by IVL (Figure 5). The minimal stent area was measured as 7.9mm2, which represented a significant increase compared to the baseline minimal lumen area. The subsequent clinical evolution was uneventful.
 Case discussion
Case discussion
This case illustrates the challenges in treating coronary artery calcified nodules. This pattern could be identified in up to 30% of patients2 and represents up to 12% of calcified stenoses.3 The presence of calcified nodules has been reported as risk factor for poor stent expansion and impaired outcomes, thus requiring adequate and dedicated plaque preparation.4,5 However, the optimal strategy remains debated in this situation.6 The use of conventional non-compliant balloons could appear as a simple first-line strategy, but is frequently inefficient for obtaining a correct pre-stent implantation result (as witnessed by a residual imprint on balloon <30%). The abrasive tools such as rotational or orbital atherectomy have an uncertain impact on this type of lesion, as they might not prepare/debulk correctly the most eccentric portion of the calcified stenosis and could, in addition, damage the healthy portion of the vessel. This issue could be very relevant in case of tortuous vessel or ostial lesion, which represents per se an independent risk factor for increased major adverse cardiovascular events (MACE) over time in the case of rotational atherectomy use.5
Contrastingly, IVL therapy has been reported to be safe and equally efficient in calcified nodules and non-calcified nodule lesions (according to the post stenting minimal lumen area measurements) in the DISRUPT-CAD III pooled data analysis.3 Moreover, the most recent data from this cohort reported comparable two-year clinical outcomes in patients with or without initial calcified nodules.7 Hence, although the maximal benefits of IVL have been presumed to be observed in cases of concentric/calcified ring lesions,6 these results suggest that IVL could be considered in cases of heavily calcified nodules. In our case, IVL also appeared as a safer approach than rotational atherectomy in this ostial lesion.5
Calcified nodules are frequently associated with diffuse vessel calcifications that can be easily identified by intracoronary imaging.2 Thus, calcified nodules represent the visible luminal “tip” of an underlying severe calcified atherosclerosis “iceberg”. This implies that the vessel preparation before stenting should be considered in a greater and longer portion (surrounding lesions) than just the most stenotic lesion. In this perspective, the introduction of the new Shockwave C2+ catheter represents a more appropriate option than the previous C2 device: as the number of delivered pulses is now 120 (compared to 80), it allows the treatment of a longer segment through a more intense focused therapy on the calcified nodule until the optimal result, the disappearance of any residual imprint, is achieved. Interestingly, previous experience of calcified nodule IVL therapy with the Shockwave C2 catheter revealed that the post-stenting minimal stent area was not measured on the site of the maximal calcification but rather on the surrounding zones, suggesting the need of an extensive preparation for these segments.3 This approach and the optimal balance in the required pulses number between the focal “culprit lesion” and “associated lesions” therapy might vary according to the morphology (thickness, eccentricity) and the location of the calcified stenosis. In our patient, we delivered 50 pulses on the RCA ostium because the radiographic continuity between aortic wall and coronary calcifications suggested a massive calcium burden at this precise site. Whether this tailored IVL with the C2+ catheter could improve the therapy efficiency has to be further investigated.
In conclusion, calcified nodules are frequently identified among calcified lesions and represent a challenge for optimal PCI. The use of C2+ IVL catheter represents a valuable option for preparing the lesion and obtaining an optimal result in this situation.
References
1. Lee JB, Mintz GS, Lisauskas JB, et al. Histopathologic Validation of the Intravascular Ultrasound Diagnosis of Calcified Coronary Artery Nodules. American Journal of Cardiology, 2011. 108(11): p. 1547-1551.
2. Xu Y, Mintz GS, Tam A, et al. Prevalence, Distribution, Predictors, and Outcomes of Patients With Calcified Nodules in Native Coronary Arteries. Circulation, 2012. 126(5): p. 537-545.
3. Ali ZA, Kereiakes D, Hill J, et al. Safety and Effectiveness of Coronary Intravascular Lithotripsy for Treatment of Calcified Nodules. JACC Cardiovasc Interv, 2023.
4. Zhang M, Matsumura M, Usui E, et al. Intravascular Ultrasound Derived Calcium Score to Predict Stent Expansion in Severely Calcified Lesions. Circulation: Cardiovascular Interventions, 2021. 14(10): p. e010296.
5. Morofuji T, Kuramitsu S, Shinozaki T, et al. Clinical impact of calcified nodule in patients with heavily calcified lesions requiring rotational atherectomy. Catheter Cardiovasc Interv, 2021. 97(1): p. 10-19.
6. Shah M, Najam O, Bhindi R, et al. Calcium Modification Techniques in Complex Percutaneous Coronary Intervention. Circulation: Cardiovascular Interventions, 2021. 14(5): p. e009870.
7. Shlofmitz RA, Saito S, Honton B, et al. CRT-100.36 Impact of Calcified Nodules on 2-Year Clinical Outcomes After IVL-Assisted Coronary Stenting: Pooled Analysis From the DISRUPT CAD OCT Sub-Studies. JACC: Cardiovascular Interventions, 2023. 16(4_Supplement): p. S1-S1.










