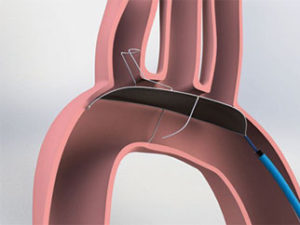
Preliminary real-world findings, presented at PCR London Valves (18–20 September, London, UK), indicate that the use of the cerebral protection device (TriGuard, Keystone Heart) in patients undergoing transcatheter aortic valve implantation (TAVI) is associated with a significant reduction in the number of brain lesions. The aim of the study was to evaluate the safety and effectiveness of the TriGuard embolic deflection device in protecting the brain from lesions during TAVI procedures compared to the current standard of care in the US and Europe, which is no cerebral protection during these procedures.
In this real world study, physicians from two institutions enrolled a total of 51 patients undergoing TAVI (80± 8 years, 51% male, logistic EuroSCORE 12.6±8.3) with either transfemoral (96%) or transapical (4%) insertion. Peter R Stella (Department of Cardiology, University Medical Centre Utrecht, Utrecht, The Netherlands) enrolled 41 patients and Joachim Schofer (Hamburg University Cardiovascular Center and Department of structural heart disease, Hamburg, Germany) enrolled 10.
The 10 enrolled by Schofer were also tested with post TAVR MRI using clinical criteria and were compared to 150 historical controls from the same institution. According to a press release, based on these MRI evaluations, results demonstrated a significant difference in the number of patients with brain lesions incurred during the TAVI procedure with 68% of unprotected patients experiencing new lesions compared to only 20% of patients for which the TriGuard embolic deflection device was used (p=0.004). Additional endpoints demonstrated clear improvement in the mean number of new lesions (2.1 for unprotected historical controls vs 0.06 for protected patient; p= 0.06), mean total lesion volume (206mm3 for unprotected historical controls vs 44mm3 for protected patients; p=0.08) and mean single lesion volume (74 mm3 for unprotected historical controls vs. 15 mm3 for protected patients; p=0.05). Of the 51 patients protected by the TriGuard device in this study, none experienced strokes, and a successful device performance of 100% was observed without interference during TAVI procedures.
Alexandra J Lansky (Division of Cardiology, Yale School of Medicine and Yale Cardiovascular Research Group, New Haven, USA), says: “These data, together with previously reported positive safety, and clinically meaningful outcomes, reinforce the importance of using TriGuard to protect the brain from damage potentially incurred during TAVI procedures.”
The press release reports that TriGuard is a cerebral embolic protection device designed to reduce the amount of embolic material that enter blood circulation to the brain during transcatheter heart valve replacement or implantation. It adds that TriGuard is the only device designed to provide full coverage of all brain territories without the need for third access site during TAVI.
Also at PCR London Valves conference, Lansky is presented an update on NeuroARC—an initiative aimed at establishing standardised neurologic endpoints for cardiovascular clinical trials to ensure clinically meaningful patient outcomes and improve the quality of clinical research. The initiative is spearheaded by a diverse working group comprised of physician and scientific leaders in interventional and structural cardiology, cardiac surgery, neurology, neuroradiology, neuropsychology, as well as clinical trialists representing academic research organizations from the US and Europe, and representatives from the medical device industry and the FDA. She comments: “Neurologic damage due to TAVI is overlooked, and protecting the brain has become a priority to improve our patients’ outcomes. Consensus-driven definitions of neurologic measures will facilitate more informed benefit-risk assessments for all procedures and devices, and improve our care of patients.”
The risk of stroke in patients undergoing TAVI