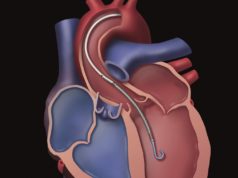
Abiomed has expanded its FDA pre-market approval for Impella heart pump use in high-risk percutaneous coronary interventions (PCI) to include its Impella CP heart pump. A press release reports that the Impella heart pumps provide the “only minimally invasive treatment option with the unique ability to stabilise the patient’s haemodynamics and unload the left ventricle of the heart”, which it adds allows the muscle to rest and recover its native function.
According to the press release, this year, Impella CP’s first FDA PMA approval was for up to four days of use to treat patients suffering from cardiogenic shock and is identical to Impella 2.5 (four days) and Impella 5.0 (six days) indications.
The Impella 2.5, Impella CP, Impella 5.0 and Impella LD catheters, in conjunction with the automated Impella controller, are temporary ventricular support devices intended for short-term use (<four days for the Impella 2.5 and Impella CP, and <six days for the Impella 5.0 and Impella LD) and indicated for the treatment of ongoing cardiogenic shock that occurs immediately (<48 hours) following acute myocardial infarction or open heart surgery as a result of isolated left ventricular failure that is not responsive to optimal medical management and conventional treatment measures, including volume loading and the use of pressors and inotropes, with or without an intra-aortic balloon pump (IABP). The intent of the Impella system therapy is to reduce ventricular work and to provide the circulatory support necessary to allow heart recovery and early assessment of residual myocardial function.
The expanded approval for Impella CP means it is identical to Impella 2.5. The Impella 2.5 and Impella CP are temporary (≤ six hours) ventricular support systems indicated for use during high risk PCI performed in elective or urgent hemodynamically stable patients with severe coronary artery disease and depressed left ventricular ejection fraction, when a heart team, including a cardiac surgeon, has determined high risk PCI is the appropriate therapeutic option. Use of the Impella 2.5 and Impella CP in these patients may prevent hemodynamic instability which can result from repeat episodes of reversible myocardial ischemia that occur during planned temporary coronary occlusions and may reduce peri- and post-procedural adverse events.
Jeffrey W Moses (Columbia University Medical Center, New York, USA), comments: “This latest approval for Impella expands the haemodynamic options for the cardiovascular community to effectively revascularise severely ill patients who have limited options and high mortality risk. Backed by clinical data and real world experience since 2008, interventional cardiologists working with their heart teams to identify complex PCI candidates can perform complete revascularisation on previously untreatable patients to improve their quality of life and their native heart function.”