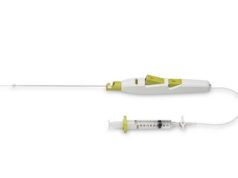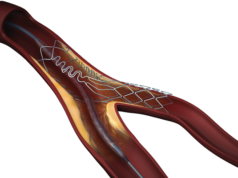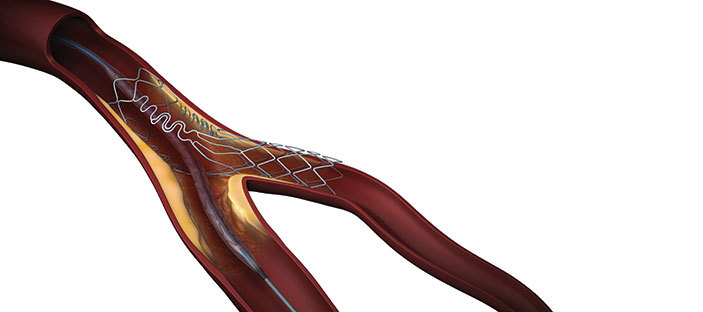
Tryton Medical has announced that the first US commercial case using the Tryton Side branch stent to treat a coronary bifurcation lesion involving a large side branch (appropriate for a ≥2.5mm stent) was completed at New York-Presbyterian Hospital/Columbia University Medical Center in New York City, USA.
The procedure was performed by Martin Leon, director of the Center for Interventional Vascular Therapy, and Ajay Kirtane, director of the Cardiac Catheterization Laboratory.
The Tryton Side branch stent recently became the first dedicated bifurcation device to receive regulatory approval in the US. Tryton has signed a strategic distribution agreement with Cardinal Health enabling Cordis, its interventional vascular business, to be the exclusive distributor of the Tryton Side branch stent in the USA.