Tag: Cordis
MedAlliance to be acquired by Cordis
MedAlliance is to be acquired by Cordis, it was announced today, after the two companies reached agr...
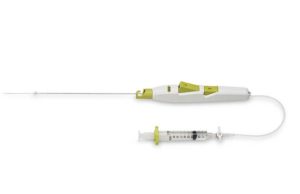
Cordis launches Mynx Control vascular closure device in the USA
Cordis, a Cardinal Health company, has launched its Mynx Control vascular closure device onto th...
First US commercial implants of Elunir drug-eluting stent performed
Cordis and Medinol have announced that the first US commercial cases using the Elunir drug-eluti...
Elunir drug-eluting stent approved for use in the USA
Cordis and Medinol have announced that the US FDA has approval the Elunir drug-eluting stent for...
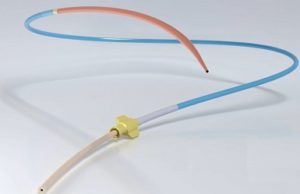
Cordis granted CE mark for Railway sheathless access system
Cordis has received the CE mark for its new Railway sheathless access system, which is now avail...
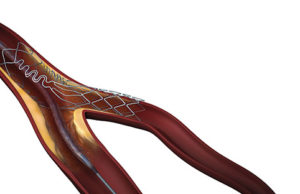
First US commercial case performed using Tryton Side branch stent
Tryton Medical has announced that the first US commercial case using the Tryton Side branch sten...
FDA grants approval to Tryton for side-branch stent
The US Food and Drug Administration (FDA) has approved Tryton Medical’s Premarket Approval (PMA)...