The idea of a fully bioresorbable scaffold is attractive as the need for mechanical support for the healing artery after percutaneous coronary intervention (PCI) is only temporary and there are potential disadvantages of a permanent metallic prosthesis. However, recent data—the three-year results of ABSORB II—indicate that the most well-known bioresorbable scaffold (Absorb, Abbott Vascular) may not provide the advantages that it initially promised. Jose Ribamar Costa Jr and Alexandre Abizaid discuss the potential reasons for this finding and review the limitations that scaffolds need to overcome if they are to replace the current generation of drug-eluting stents as the gold standard.
The results of the ABSORB A and B cohorts—the first-in-human studies with Absorb—were promising. They showed sustained neointimal hyperplasia suppression up to five years and unique features related to the scaffold resorption process, such as plaque stabilisation/regression and late lumen enlargement—not possible after the vessel caging with metallic devices. The device, following further studies, was subsequently approved for use both in Europe and in North America.
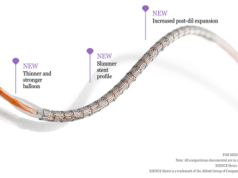
However, Absorb has now been deployed in more cumbersome clinical and angiographic scenarios and this had caused old and new enemies to arise. Notably, increased thrombosis rates, especially in the first few months after PCI, have been reported. Most of these findings have been attributed to the device intrinsic properties, including larger strut thickness (150mm) and relatively low resistance to overexpansion (which might result in scaffold fracture). Additionally, problems related to the deployment technique, including insufficient lesion preparation and low rates of scaffold post dilation (probably due to the fear of device structural damage), may have contributed to the occurrence of this serious untoward event.
The deployment technique has, therefore, been reviewed, with the emphasis on more “aggressive” lesion preparation and on high pressure post dilation with non-compliant balloons (respecting the 0.5mm expansion range of the scaffold’s instructions for use) as well as a more liberal use of intravascular imaging to guide and optimise the procedure. Consequently, more recent “real-world” registries have started to show a reduction in the acute/subacute scaffold thrombosis rates.
Meanwhile, long-term data (³24 months) from randomised, controlled trials for these novel devices have started to be presented and the negative results have surprised the interventional cardiology community. Among these studies, two should be highlighted: ABSORB Japan and ABSORB II.
In the ABSORB Japan trial, 400 patients were randomised in a 2:1 ratio to Absorb (n=266) or Xience (Abbott Vascular; n=134). Although both groups had low and similar rates of device failure and thrombosis at one year, there was a trend towards more target lesion failure with Absorb (7.3% vs. 3.8% for Xience; p=0.18) at 24 months. Furthermore, very late scaffold thrombosis (beyond one year) was observed in 1.6% (n=4) of patients the Absorb arm but not at all in patients in the Xience arm.
More recently, Patrick Serruys (Faculty of Medicine, National Heart & Lung Institute, Imperial College, London, UK) presented, one behalf of his fellow investigators, the three-year results of the ABSORB II trial at the 2016 Transcatheter Cardiovascular Therapeutics (TCT) meeting (29 October—2 November, Washington DC, USA). The trial, which was simultaneously published in The Lancet, randomly assigned patients (2:1) to receive Absorb (n= 335) or Xience (n=166). The aim of primary endpoint was to demonstrate two mechanistic properties of bioresorbable scaffolds: increase in luminal dimensions as a result of recovered vasomotion of the scaffolded segment. However, the vasomotor reactivity at three years was not statistically different (0.047mm±0.11mm for Absorb vs. 0.056mm±0.12mm for Xience; p=0.49). Furthermore, a device-oriented composite endpoint was significantly increased in the Absorb arm (10% vs. 5% for Xience; p=0.0425). This result was mainly driven by target vessel periprocedural myocardial infarction in the Absorb arm (4% vs. 1%, respectively; p=0.16). The definite/probable thrombosis rate was also higher in this cohort (3% vs. 0%; p=0.033), with most of the cases being very late scaffold thrombosis (six out of nine cases).
While the acute problems observed with bioresorbable scaffold could be attributed to modifiable properties of the scaffolds (eg. strut thickness, resistance to oversizing, inadequate deployment technique, etc.), the more recent negative findings defy the logic of resorption and pose a challenge to the whole concept behind the bioresorbable scaffold technology. It is not yet completely understood why, in ABSORB II, the late vasomotion in the Absorb group was similar to that of the Xience group (where the stented segment is caged by the metallic struts of the device) given that the Absorb struts should have been either absorbed or should be at an advanced level of resorption where theoretically no mechanical property was detected.
Furthermore, in the ABSORB Japan trial, in most cases of very late scaffold thrombosis (three out of four), optical coherence tomography (OCT) at the time of or shortly after the event demonstrated strut discontinuities, malapposition and/or uncovered struts. This is somewhat unexpected based on initial pre-clinical and clinical data with these devices, which showed an early and homogenous strut healing and lack of mechanical properties after the first 12 months of the procedure.
A possible explanation to these negative observations is the difference between the absorption time in vitro and that of in vivo, with the process perhaps more delayed in humans than foreseen from the preclinical studies. Also, the magnitude of the resorption process, generating local inflammatory reactions, should play a role on these findings. Finally, the current generation of metallic drug-eluting stents has also experienced huge improvements, with the introduction of more biocompatible and even biodegradable polymers and thin strut platforms (<90mm), which allow excellent acute performance even in more challenging scenarios and very long-term sustained safety and efficacy. In other words, now the bar is now set “very high” for novel technologies.
Therefore, the future of the bioresorbable scaffold technology as the “working horse” in the interventional cardiology field lays on the clinical performance of the next generation of these devices. To overcome these shortcomings, the second-generation of bioresorbable scaffolds need to be mechanically stronger, have thinner struts with better crossing profile, be available in a broader range of length and diameter. On the positive side, these devices are rapidly being iterated to improve deliverability and ease of use and to enhance safety and efficacy profile. Various post-processing methods of polymers are being explored and investigated (extrusion, annealing, electro spinning, microfiber and micro braiding) to overcome current limitations.
J Ribamar Costa Jr is at Instituto Dante Pazzanese de Cardiologia, Hospital do Coração and Associação do Sanatorio Sírio (both São Paulo, Brazil), and Alexandre Abizaid is at Instituto Dante Pazzanese de Cardiologia, Hospital do Coraçã, Associação do Sanatorio Sírio, and Hospital Sirio Libanês (all São Paulo, Brazil)