Tag: Abbott Vascular
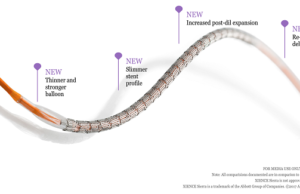
Xience Sierra is now on European market
The CE mark has been awarded to a new-generation of Abbott Vascular's everolimus-eluting, metall...
“Low commercial sales” prompt Abbott to pull the plug on selling Absorb
Abbott has announced that it is to stop selling its first-generation bioresorbable vascular scaf...
New study to evaluate safety of three-month DAPT with Xience in high bleeding risk patients
A new study, XIENCE Short DAPT, will evaluate whether three months of dual antiplatelet therapy ...
EuroPCR 2017: Study finds no association between implantation technique and risk of scaffold thrombosis
A study—AIDA (Amsterdam investigator-initiated Absorb strategy all-comers trial)—presented at Eu...
Absorb GTS to be restricted to registry use only in Europe
Abbott Vascular has written a letter to European physicians to say that from 31 May, its bioreso...
ACC 2017: Two-year rate of target lesion failure significantly higher with Absorb
Two-year data from the ABSORB III study indicate that a bioresorbable vascular scaffold (Absorb,...
The implications of ABSORB II three-year results for the future of bioresorbable scaffolds
The idea of a fully bioresorbable scaffold is attractive as the need for mechanical support for ...
Combining MitraClip mitral valve repair with left atrial appendage occlusion is safe and feasible
Shingo Kuwata (University Heart Center, Zurich, Switzerland) and others report in Eurointerventi...
St Jude Medical shareholders approve merger with Abbott
St Jude Medical has announced that, based on the preliminary voting results from St Jude Medical’s A...
PCRLV 2016: MitraClip may be “safe and feasible” for treating tricuspid regurgitation
A first-in-man study indicates that percutaneous edge-to-edge repair with Abbott Vascular’s Mitr...