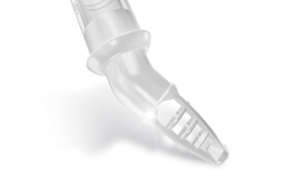
LivaNova has received FDA 510(k) clearance for the US market launch of its Optiflow arterial cannulae family. A press release reports that the Optiflow aortic arch cannulae provide improved hydrodynamics with a novel dispersive tip design that improves blood flow characteristics resulting in reduced wall shear stress profiles.
The press release states that unlike standard arterial cannulae, Optiflow arterial cannulae feature a unique basket tip with large openings that allow a more physiologically compatible dispersive design. It adds that this design has been shown to significantly reduce wall shear stress and turbulence, thereby improving hydrodynamics and potentially reducing ischaemic complications from extracorporeal circulation (ECC) during cardiac surgery.
In a recent trial using advanced numerical evaluation of a human aorta model and its branches simulating the effect of aortic cannulae on blood flow characteristics, Assmann et al demonstrated a 50% decrease in average and peak wall shear stress during both pulsatile and nonpulsatile ECC with dispersive cannula tips compared to standard cannula tips.
Alexander Assmann (Heinrich Heine University, Düsseldorf, Germany) comments: “The sandblasting effect of conventional arterial ECC cannulae can result in endothelial damage and plaque mobilization, and arterial embolisation of atherosclerotic plaques during ECC surgery frequently results in severe complications such as stroke or ischemia of other organs. The remarkable decrease in wall shear stress profiles and turbulence using dispersive cannula tips presents surgeons with the opportunity to reduce risk and improve outcomes when ECC is employed during cardiac surgery.”