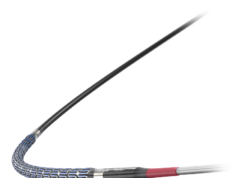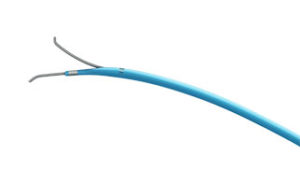
FineDuo—a low profile, multifunctional, dual-lumen microcatheter—now has the CE mark. The device has been developed for use during percutaneous transluminal coronary angioplasty for guidewire support for crossing side branch and exchange of guidewires. According to a press release, it provides provide robust support and easy access to complex coronary vasculature.
The press release also reports that the FineDuo microcatheter allows the operator to deliver a second guidewire through the over-the-wire lumen while leaving the first guidewire in the monorail lumen in place. Terumo expects to launch the FineDuo dual-lumen microcatheter in Europe in October 2016.
The launch follows an original equipment manufacturer agreement on dual-lumen microcatheters for European markets between Kaneka and Terumo. Kaneka will start to supply its dual-lumen microcatheters—named Crusade—to Terumo, who are selling it as FineDuo. Kaneka will continue to sell it as Crusade dual-lumen microcatheter.
Terumo will be exhibiting at the Euro CTO Club (30 September–1 October, Krakow, Poland) and at the European Bifurcation Club meeting (14–15 October, Rotterdam, The Netherlands).