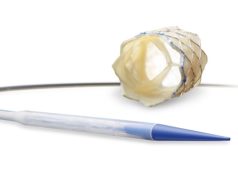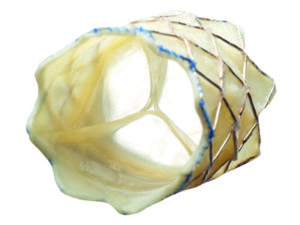
Following FDA approval, Medtronic’s Melody transcatheter pulmonary heart valve can now be used to treat patients whose surgical bioprosthetic pulmonary heart valves have failed. A press release reports that the valve is specifically designed for use in the pulmonary position and is the first transcatheter pulmonary valve to receive this approval in the USA.
During a valve-in-valve procedure, the Melody valve is placed inside a failing pulmonic surgical heart valve through the recently launched Ensemble II Delivery System, a low-profile, delivery catheter, specifically designed to deliver the Melody device.
Jeremy Asnes (Congenital Cardiac Catheterization Laboratory, Yale School of Medicine, New Haven, USA), says: “As the first commercially available transcatheter heart valve, the Melody transcatheter valve brought a breakthrough non-surgical option to treat failing pulmonary valve conduits. Thousands of congenital patients globally have benefited from this therapy in the past decade. With this expanded indication, we can further reduce the need for obtrusive open-heart surgery and allow even more patients to benefit from this minimally invasive treatment option.”