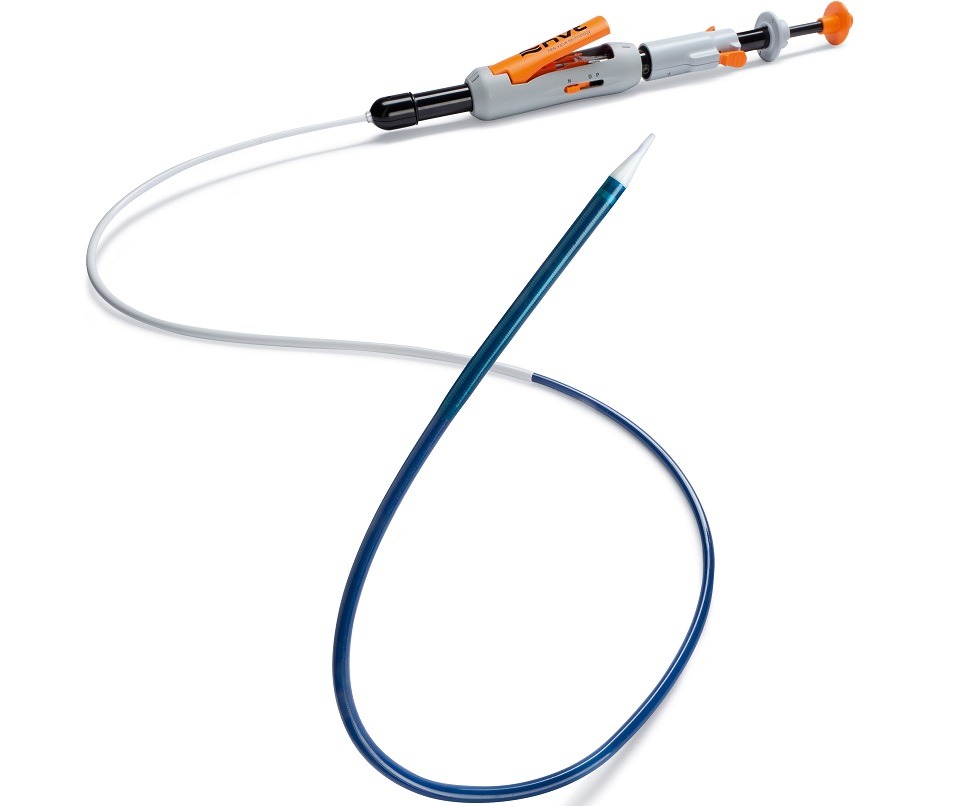
NVT has received the CE mark for its Allegra transcatheter aortic valve, which it describes as “distinctive” in a company release. The product features a low-profile transfemoral delivery system.
The Allegra transcatheter aortic valve implantation (TAVI) system is approved for the treatment of severe calcified aortic valve stenosis in high-risk patients with elevated surgical risk. It consists of a transcatheter heart valve (THV) in three different sizes with an annulus size ranging from 19mm to 28mm, a delivery system, and a loading system.
Allegra is designed to improve the long-term survivability of patients by resolving the clinical issues associated with current commercial valves. Three sizes of valves—23mm, 27mm and 31mm—will be commercially available in Europe immediately, all delivered via a flexible, 18 French transfemoral delivery system with an integrated sheath intended to provide a stable implantation. The system is also designed to reduce procedural risk by aiming to eliminate the need for rapid pacing during deployment.










