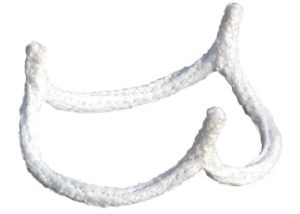
BioStable Science & Engineering has completed a direct de novo application to the FDA for Class II designation of its HAART 300 aortic annuloplasty device. A press releases states that the submission of the de novo request is an important milestone in BioStable’s efforts to bring the potential benefits of valve repair to US patients with aortic valve insufficiency.
According to the press release, implantation of the HAART 300 aortic annuloplasty device is designed to conform the aortic annulus to the three-dimensional shape of the device, thereby potentially restoring and maintaining more normal anatomical valve geometry for proper valve closure. Once implanted, the HAART 300 device serves as a framework for completing the overall repair procedure to maintain the patient’s native valve.
After receiving the CE mark in March 2016, the HAART 300 aortic annuloplasty device became the first commercially available internal annuloplasty device developed for aortic valve repair. A limited commercial launch was initiated in May in the EU, and a select number of European heart centres have already completed training and are incorporating the device within their valve repair programmes.
Commenting on the company’s recent accomplishments, John Wheeler, BioStable’s President and chief executive officer, says: “The HAART aortic repair technologies are design to make surgical repair of the aortic valve a simpler and more standardised procedure. Submission of the HAART 300 de novo application and completion of the HAART 200 clinical study are important achievements towards BioStable’s goal of expanding the availability of our HAART aortic repair technologies for surgeons and patients.”
The company recently concluded the European multicentre clinical study of the HAART 200 aortic annuloplasty device for bicuspid valve repair. The two-year outcomes from the HAART 200 study will be used to support an application for CE mark in early 2017.









