A novel device for treating tricuspid regurgitation has become the 2016 recipient of the European Association for Cardio-Thoracic Surgery (EACTS) Techno College Innovation Award. The award, which is chosen by the EACTS New Technology Committee, was presented during the society’s annual meeting (1–5 October, Barcelona, Spain). Maximilian Kütting of NVT GmbH, the company that developed the winning system, speaks to Cardiovascular News about the device.
Why is there a need for novel therapies for managing tricuspid regurgitation?
A large number of patients suffer from tricuspid regurgitation. When it has progressed to a severe or massive state, tricuspid regurgitation not only affects quality of life but also significantly worsens survival.
Furthermore, the mortality after surgical correction of tricuspid valve regurgitation is relatively high—especially in patients undergoing redo-operations. These high risk patients are often considered inoperable and their only option is medical therapy. Considering transcatheter options for aortic and mitral valve conditions are now being clinically applied, patients who suffer from secondary tricuspid regurgitation resulting from left sided heart disease require new treatment options to complete the therapy.
How does your device work?
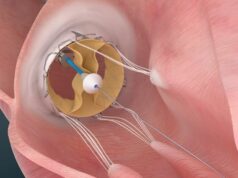
The device consists of a stentgraft with a lateral bicuspid valve, both made from nitinol and porcine pericardium. The idea is to focus on the alleviation of symptoms that patients with tricuspid valve regurgitation experience (such as ascites and venous congestion) that are caused by backflow into the venous system. This backflow is eliminated by placing our device and, thus effectively, a fifth heart valve into the right atrium. The stentgraft serves as the sealing and anchoring element and additionally has the benefit of excluding a part of the right atrium and thereby reducing its volume.
Our collaboration with the company JOTEC—who have tremendous experience in the field of stentgraft development and delivery—as well as our experience of developing a transcatheter aortic valve (TAVI) prosthesis (NVT Allegra) has helped us to develop this device. Since the device does not interfere with the tricuspid annulus, it does not depend on annular dimensions and could also be placed in patients with a failed previous repair of the tricuspid valve.
Are you able to use your system in combination with transcatheter mitral therapies?
The combined treatment of mitral and tricuspid conditions will be necessary for a lot of patients undergoing tricuspid valve procedures.
The design of the device does not impede transseptal access to the left atrium. Later transapical or direct transatrial implantation of devices will also not face any interference with our device. At this stage of the development of transcatheter mitral valves, it is difficult to predict which access routes will be used and each procedure must be analysed individually, but there are no general exclusions or contraindications.
What data are available for your system?
We currently have in-vitro data that demonstrates the performance of the device both in terms of hydrodynamics and durability. There are also the encouraging results from animal studies in an ovine model as well as human cadaver studies that we performed together with Henning Lausberg and Christian Schlensak (both University Clinic of Tübingen, Germany). While no clinical studies with the system have been performed to date, we aim to achieve this within the coming months.
Looking at clinical experience with similar technologies, caval vein implantation comes closest and while there is only limited experience, the therapeutic effects were demonstrated and appear promising. Our technology further simplifies the implantation procedure while also eliminating backflow into both caval veins.
What studies are planned?
Our first aim is to show the feasibility of the concept in the planned patient population in combination with optimal medical therapy to maximise the effect of the treatment for the patient. It will be critical to learn which patients with tricuspid regurgitation benefit most from this form of treatment. The prosthesis will initially only be implanted in patients who are considered inoperable by surgeons and respond poorly to medical therapy. We will then build on this experience and possibly expand the indication according to outcomes. The focus is primarily on the reduction of symptoms of tricuspid regurgitation and subsequently an improvement in the patient’s quality of life.