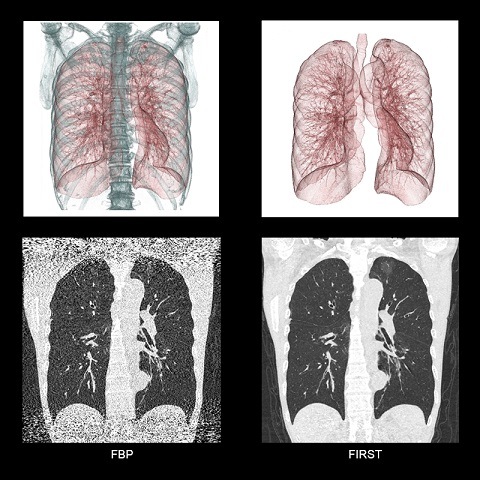
Toshiba Medical’s Forward projected model-based iterative reconstruction solution (FIRST) and Vantage Titan 1.5T/cS edition with M-Power v3.6 software have received US Food and Drug Administration (FDA) clearance.
FIRST is designed to improve high-contrast spatial resolution, while reducing radiation dosage by up to 85.3% as compared to filtered back projection. According to a company release, the product drastically cuts the time needed for model-based computed tomography (CT) image reconstruction.
“FIRST produces images that are natural-appearing and processes them fast enough for routine clinical work,” says Marcus Chen, director, Cardiovascular CT, National Heart, Lung and Blood Institute, National Institutes of Health (Bethesda, USA), who was part of the beta testing for FIRST. “With FIRST, we can improve image quality while lowering radiation dose.” NHLBI has a research agreement with Toshiba Medical to help develop and test new technologies for CT.
According to the company release, FIRST can improve high-contrast spatial resolution by up to 129%, as compared to filtered back projection for body imaging, while reducing radiation dose. The can be integrated into scan protocols adapting to the individual patient and clinical task at hand, requiring as little as three minutes for volumetric reconstructions, the release reports.
Toshiba’s Vantage Titan 1.5T/cS edition with M-Power v3.6 software includes technology designed to simplify complex cardiac exams:
- Surevoi Cardiac anatomy recognition technology is designed to automatically determine the position of the heart.
- The enhanced CardioLine application, CardioLine+, is intended to work with Surevoi to automatically detect anatomical landmarks for accurate and reproducible positioning to complete alignment of 14 standard cardiac views with minimal operator interaction.
- The Vantage Titan/cS Edition allows acquisition of multiple b-values in a single scan, intended to enable physicians to identify tissue diffusivity quickly.
- The system also supports Multi-echo T2 Mapping, allowing physicians to visualise T2 map cartilage images to aid them in making a diagnosis and determining if treatment is needed.
- Vitrea Extend, a post-processing application, is intended to allow clinicians to complete post-processing at a separate workstation while allowing the next patient exam to be conducted at the MR system. The Vitrea Extend solution comes with expert packages powered by the latest post-processing applications made by Olea Medical and Medis.












