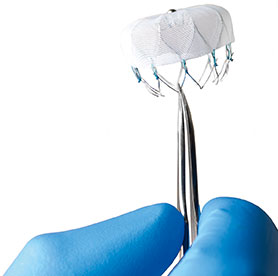
An American College of Cardiology (ACC) press statement reports that the Centers for Medicare and Medicaid Services (CMS) has identified the LAAO Registry as an approved registry to meet the requirements of the national coverage determination (NCD) for Medicare patients undergoing percutaneous left atrial appendage closure.
Launched in December 2015, according to the ACC press statement, the LAAO Registry is the first national registry capturing data on left atrial appendage occlusion procedures to assess real-world procedural outcomes, short and long-term safety, comparative effectiveness and cost effectiveness. Patient-level data will be submitted by participating hospitals on a quarterly basis to ACC’s National Cardiovascular Data Registry (NCDR). Physicians and hospitals can use these data to support quality improvement efforts locally and monitor patterns of care. Registry data will be used to help develop clinical guidelines leading to improved patient care. Ultimately, the registry will provide a better understanding of this population and emerging treatment options.
For more information, click here












