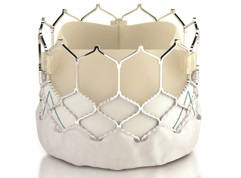
Edwards Lifesciences has received US Food and Drug Administration (FDA) approval for aortic and mitral valve-in-valve procedures using the Edwards Sapien 3 transcatheter heart valve. According to a company release, the Sapien 3 valve is the first transcatheter heart valve approved in the USA for the treatment of both aortic and mitral patients who are at high risk for a subsequent open-heart surgery to replace their bioprosthetic valve.
FDA approval of the indication expansion was supported by real-world data collected from the Society of Thoracic Surgeons and American College of Cardiology (STS/ACC) Transcatheter Valve Therapy (TVT) Registry. The TVT Registry includes information and outcomes on patients undergoing transcatheter valve replacement and repair procedures in the USA.
“This approval brings a safe and effective transcatheter therapy to patients who would do very poorly with repeat open-heart surgery,” says John Carroll, professor of cardiology at the University of Colorado School of Medicine and director of interventional cardiology at the University of Colorado Hospital, Denver, USA, and member of the TVT Registry Steering Committee. “I am pleased to see that the FDA recognises the value of the high-quality evidence generated by the STS-ACC TVT Registry and its ability to play an important role in assessing ‘real-world’ clinical results in specialty indications, such as valve-in-valve, and for particular patient groups, such as those needing replacement of a bioprosthetic mitral valve.”
The Edwards Sapien 3 valve was approved by the FDA in 2015 for severe, symptomatic aortic stenosis patients at high risk for open-heart surgery, and, in 2016, received approval for the treatment of patients who are at intermediate risk for open-heart surgery.













