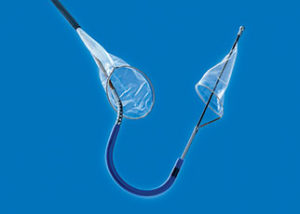
Claret Medical has announced its filing of a marketing application with the FDA for clearance of the Sentinel cerebral protection system. According to a press release, Sentinel is the only device designed to protect the brain by capturing and removing debris dislodged during transcatheter aortic valve implantation (TAVI). There are currently no cerebral embolic protection technologies available in the US to protect TAVI patients from cerebral embolic events.
The press release reports that the filing includes data from the recently completed SENTINEL pivotal IDE trial, a prospective, randomised, controlled, blinded study of 363 TAVI patients at 19 centres in the US and Germany. Trial endpoints included reduction in new ischaemic cerebral infarcts, major adverse cardiac or cerebrovascular events, neurocognitive outcomes, and qualitative and quantitative histopathological findings. The SENTINEL trial allowed inclusion of all commercially available TAVI platforms available in the US.
Results from the recently published CLEAN-TAVI blinded, randomised, controlled trial in the Journal of the American Medical Association (JAMA) and the MISTRAL-C randomised, controlled trial, published in Eurointervention, showed that patients protected with the system had significantly fewer and smaller new ischaemic cerebral infarcts following the procedure than unprotected patients. MISTRAL-C also demonstrated that the TAVI procedure created embolic debris in 100 percent of patients, which could have travelled to the brain if not for the protection offered by Sentinel.
Claret Medical President and chief executive officer Azin Parhizgar comments: “Our contribution in building significant new science will help the rapidly growing TAVI field embrace the critical role of cerebral protection in all left heart and endovascular procedures. These studies incorporate a level of rigorous brain evaluation never before undertaken, including systematic and serial state-of-the-art 3-Tesla MRI neuroimaging of patients’ pre-existing cerebrovascular disease, as determined by a baseline MRI, as well as neurological, histopathological and neurocognitive evaluations. Assuming a positive outcome with the FDA, we look forward to making the Sentinel available to interventional cardiologists and cardiovascular surgeons in the US soon.”
The SENTINEL IDE trial is due to be presented as a late-breaking trial at the 2016 Transcatheter Cardiovascular Therapeutics (29 October-2 November, Washington DC, USA).












