Boston Scientific has announced the schedule of key data presentations, including four late-breaking clinical trials, which will be featured at the annual EuroPCR Scientific Program, in Paris, 16-19 May. The late-breaking clinical trials include the following:
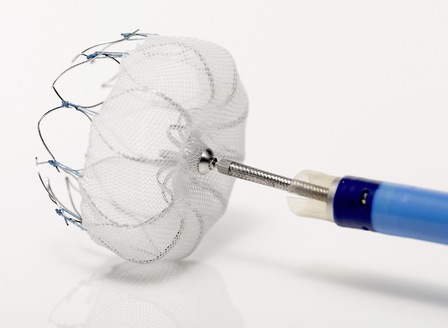
- Evaluation of 30-day safety and one-year efficacy outcomes from the REPRISE III trial, a prospective, randomised investigation of the Lotus valve system, a transcatheter aortic valve implantation (TAVI) device against the CoreValve TAVI system (Medtronic).
- The LOTUS Valve RESPOND study providing one-year, ‘real-world’ clinical and device performance outcomes data.
- Three-year outcomes of the EVOLVE II trial assessing the Synergy Bioabsorbable polymer drug-eluting stent system in patients with diabetes.
- Data from the EWOLUTION registry, a prospective multicentre study of ‘real-world’ outcomes with the Watchman left atrial appendage closure (LAAC) Device.
“This year’s EuroPCR congress will provide us the opportunity to share the performance of our Lotus valve platform in the REPRISE III trial, which is the first large, global, randomised head-to-head trial of two contemporary TAVI valves, as well as findings from the RESPOND and RESPOND Extension studies,” says Ian Meredith, executive vice president and global chief medical officer, Boston Scientific.













