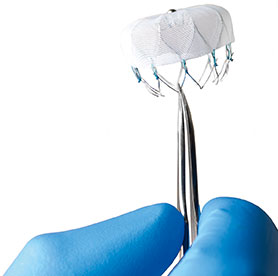
Boston Scientific has announced key data that will be presented at the 2016 Transcatheter Cardiovascular Therapeutics (TCT) meeting (29 October–2 November, Washington, DC, USA). Notably, the Watchman left atrial appendage closure device data will be featured in a late-breaking clinical trial session on 2 November and will highlight the procedural safety of the device since it became commercially available in the USA last year.
A press release reports that the PLATINUM Diversity trial, which examined acute procedural performance with the Promus Premier everolimus-eluting stent in women and minorities (historically under-represented patient populations in clinical trials), will be also presented at the first report investigation session on 1 November.
Other oral presentations of interest include PREVAIL three-year and PROTECT AF five-year follow-up data related to long-term event rates for left atrial appendage closure with the Watchman device and three-year outcomes of the Lotus valve system in high-risk patients with severe aortic stenosis.
Keith Dawkins, global chief medical officer, Boston Scientific, comments: “Over the course of my 30-year career as an interventional cardiologist and chief medical officer, there has been a renaissance in the treatment of heart disease—with a surging reliance on minimally-invasive devices to deliver the outcomes previously achieved with surgery. In addition to enhancing the established body of clinical evidence for our products which treat coronary and peripheral artery disease, this meeting will demonstrate the exceptional advancement of our structural heart portfolio—comprised of the Lotus valve system for transcatheter aortic valve implantation, and the Watchman device, the only FDA-approved left atrial appendage closure device.”












