Results of the REVELUTION study, simultaneously presented at the 2016 Transcatheter Cardiovascular Therapeutics (TCT) meeting (29 October-2 November, Washington, DC, USA) and published in the Journal of the American College of Cardiology today, indicate that a novel drug-filled stent (Medtronic) has a similar rate of in-stent late lumen loss at nine months as a zotarolimus-eluting permanent polymer stent (Resolute, Medtronic). The study, which is the first-in-human data for the drug-filled stent, also showed positive clinical outcomes.
The REVELUTION trial enrolled 101 patients at 14 centres in three countries in two different cohorts measuring outcomes at nine months and 24 months. In the first 50 patients included in the nine-month primary endpoint cohort, the drug-filled stent had in-stent late lumen loss of 0.26mm, which was non-inferior to the historical control (Resolute; p<0.001). In addition, the drug-filled stent demonstrated low rates of target lesion failure (2.1%) and zero stent thrombosis at nine months.
Optical coherence tomography (OCT) data were reported from two cohorts of patients who were scheduled to receive OCT either at one and nine months (15 patients) or three and nine months (15 patients). In both groups, the drug-filled stent showed an early healing profile with the median strut coverage (new cell growth over stent struts) of 91.4% and 95.6% at one and three months, respectively, and low rates of malapposed struts at one month (0.3%) that decreased to 0% at nine months. At nine months, the median strut coverage increased to 99.1% with no cases of late acquired stent malapposition.
Study investigator, Stephen Worthley (Royal Adelaide Hospital, Adelaide, Australia), who presented the data at TCT, says: “These early results demonstrate that the controlled and sustained elution of the polymer-free drug-filled stent show the potential for rapid healing without inflammation and positive clinical outcomes,” said “In addition, we look forward to better understanding how this technology may potentially impact the duration of dual antiplatelet therapy in some patients.”
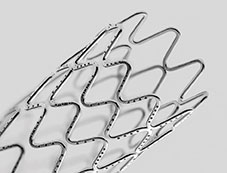
A Medtronic press release reports that the device builds on the continuous sinusoid technology platform and CoreWire technology. Introduced with the Resolute Integrity drug-eluting stent, this technology is a unique Medtronic method of manufacturing that involves forming a single strand of wire into a sinusoidal wave to construct a stent, enabling a continuous range of motion, resulting in excellent deliverability and conformability to the vessel wall. CoreWire Technology is designed to allow the stent forming wire to have multiple layers with a denser core metal surrounded by a cobalt alloy outer layer for thinner struts and greater deliverability, while offering enhanced radiopacity without compromising structural strength.













