The acquisition, which is subject to certain conditions, is expected to close in October 2015.
The supplemental New Drug Application seeks the addition of ST segment elevation myocardial infarction indication for Aggrastat.
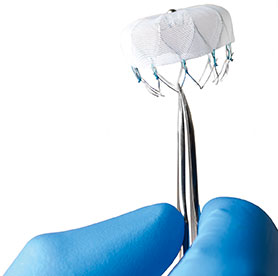
Admedus will present CardioCel data for the complete repair of aortic heart valves at the 29th European Association for Cardio-Thoracic Surgery Annual Meeting (EACTS) (3-7 October, Amsterdam, the Netherlands).
Vivid Sehgal’s appointment as chief financial officer of LivaNova will be effective with the closing of the proposed merger of Sorin and Cyberonics.
Researchers analysed data derived from the TITAN clinical trial, which demonstrated significant clinical merits of the Carillon technology, and developed a comprehensive cost-effectiveness model that projects the costs of the technology and compares them to the benefits.
The SHIELD II US clinical trial will examine the use of the HeartMate PHP in patients undergoing a high-risk percutaneous coronary intervention (PCI) procedure.
The US Food and Drug Administration (FDA) has approved AstraZenca's Brilinta (ticagrelor) tablets at a new 60mg dose to be used in patients with a history of heart attack beyond the first year.
Javier Escaned (head of Section, Interventional Cardiology Unit, Hospital Clinico San Carlos, Madrid, Spain) is a co-course director of EuroPCR (17-20 May, Paris, France).
New data indicate that the intravenous antiplatelet agent cangrelor (Kengreal, The Medicines Company) is associated with significant reduction in ischaemic events compared with clopidogrel in patients undergoing PCI with either the femoral or the radial approach.
The FDA has granted Mitralign investigational device exemption approval for the company to conduct an early feasibility study to examine its percutaneous tricuspid valve annuloplasty system. The SCOUT Study will take place in select centres in the USA.
The first patient has been enrolled in the BIOFLOW-VI clinical study in China. The aim of the study is further demonstrate the safety and efficacy of a hybrid drug-eluting stent (Orsiro, Biotronik) and support Chinese market approval.
Results from the ABSORB-Japan study, which were presented at the 2015 European Society of Cardiology meeting, indicate that the bioresorbable vascular scaffold (Absorb, Abbott Vascular) is non-inferior to an everolimus-eluting stent with a permanent polymer (Xience, Abbott Vascular).
Valtech Cardio specialises in the development of innovative surgical and transcatheter valve repair and replacement devices for the treatment of mitral valve regurgitation and tricuspid valve regurgitation.
The PLATFORM study, which was presented at the ESC, indicates that use of FFRCT is associated with a significant reduction in the number of invasive tests that do not find evidence of obstructive coronary artery disease.
The five-year results of the FAME study, which were presented at the ESC yesterday, have confirmed that FFR-guided PCI has sustained, long-term benefits compared with angiography. They show that FFR-guided PCI can contribute to reductions in all-cause mortality, cardiac mortality and an overall use of healthcare resources.
In the ALBATROSS study, which was presented at the ESC Congress this morning, adding aldosterone to standard therapy in patients with acute myocardial infarction was not associated with a significant reduction in the primary outcome (which included death, resuscitated cardiac arrest, and significant ventricular arrhythmia)
The FDA has approved evolocumab (Repatha, Amgen), which is a new cholesterol-lowering medication that inhibits proprotein convertase subtilisin/kexin type 9 (PCSK9)-a protein that reduces the liver’s ability to remove low-density lipoprotein cholesterol (LDL-C).
Philippe G̩n̩reux and others report in the Journal of the American College of Cardiology that data from ADAPT-DES indicate that the risk of all-cause mortality that is associated with post-discharge bleeding after PCI is 2.6-fold greater than the risk of all-cause mortality that is associated with post-discharge myocardial infarction.
Royal Philips has announced its presence at ESC Congress 2015, where the company is showcasing its latest cardiology solutions, including Heart ModelA.I., EchoNavigator and IntelliSpace Cardiovascular
The president of the ESC, Fausto Pinto, claims that the society is "very excited" to be holding its annual congress in London (UK) this week, stating that the city "has a fantastic reputation for welcoming people from all around the world from international sporting competitions to ground-breaking educational and medical conferences."
This European Society of Cardiology (ESC) video explores the role of comprehensive rehabilitation programmes for the secondary prevention of myocardial infarction.
Medtronic has announced that it has signed a definitive agreement to acquire the company Twelve, which is focused on the development of a transcatheter mitral valve implantation (TMVI) device.
Colibri Heart Valve has received an issue notification from the US Patent and Trademark Office regarding the forthcoming granting of a US Patent titled, "Percutaneous replacement heart valve and a delivery and implantation system."
Ferrari is currently the managing director and co-founder of De Novo Ventures and brings a wealth of strategic and operational knowledge SentreHEART's board.
Edwards will oversee all global clinical and regulatory efforts including the company's US SALUS pivotal trial studying the Direct Flow Medical Transcatheter Aortic Valve System, as well as the DISCOVER post-market European study.
The Medicines Company will present results of recent clinical studies from its cardiovascular product portfolio at the 2015 European Society of Cardiology (ESC) Congress (29 August-2 September, London, UK).
The international clinical trial TWILIGHT will test the safety and effectiveness of treating coronary stent patients with the anti-clotting medication ticagrelor instead of combining it with aspirin.
The Cardiovascular Research Foundation (CRF) has announced the late-breaking trials and first report investigations that will be presented at this years' Transcatheter Cardiovascular Therapeutics (TCT) 2015 scientific symposium.
Cyberonics has set a date for a special meeting of its stockholders to consider and vote on the transaction and certain other related matters on 22 September.
This is the first study to focus on the burden of residual angina after an initial heart attack and re-hospitalisations in patients without versus with obstructive coronary artery disease.
The editors of Annals of Internal Medicine have called for doctors to confront colleagues who act in a disrespectful manner towards patients after an anonymous essay in the journal highlighted incidences in which unprofessional behaviour was condoned.
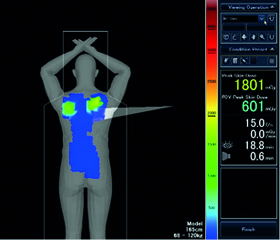
Corindus Vascular Robotics and Unfors RaySafe have announced a distribution agreement to allow Corindus to offer the RaySafe i2 real-time radiation dose monitoring system in conjunction with its CorPath system.
Abiomed has submitted US Food and Drug Administration pre-market approval supplemental submissions to expand Impella 2.5 pre-market approval to the entire Impella family of devices (Impella 2.5, Impella CP and Impella 5.0/LD).
The advent of second-generation drug-eluting stents and the associated improved safety and efficacy has meant that the risks of using two stents for bifurcation lesions has significantly decreased. Maciej Lesiak explores when a two-stent strategy should be used.
In this video for the Cardiothoracic Surgery Network, Jill Ley leads an expert panel discussion about managing patients with cardiac arrest after cardiac surgery. This video was filmed at the Society of Thoracic Surgeons' annual meeting.
Medtronic has issued a Class 1 recall of its EnVeo R loading system following eight reports of the presence of particulates, which could potentially lead to blockages in the bloodstream.
Increasing prevalence of cardiovascular diseases coupled with adoption of minimally invasive surgeries is expected to boost the interventional cardiology device market over the forecast period.
US Endovascular and BrosMed have entered into an exclusive agreement to distribute Artimes semi-compliant and Apollo non-compliant coronary angioplasty catheters.
The FDA has issued a safety alert telling healthcare professionals, patients, and carers about potential serious adverse effects with left ventricular assist devices. The alert relates to Thoratec's HeartMate II device and HeartWare' HVAD.
A study indicates that normalisation of total testosterone levels with testosterone replacement therapy is associated with a significant reduction in the risk of myocardial infarction, stroke and all-cause mortality compared with non-normalisation with testosterone replacement therapy and no therapy in men with low testosterone.
Pascal Meier believes that social media is the logical next step from the internet-just as the internet has revolutionised connectivity, social media is revolutionising communication. In this commentary, he explains why social media presents a great opportunity for interventional cardiologists.
A press release says that the combined company will leverage the technical expertise of both NDC and Interface in highly precise and demanding medical devices.
The Tryton registry is designed to confirm the results from Tryton's pivotal Investigational Device Exemption (IDE) trial, and has successfully enrolled 133 patients from Europe and the USA.
In this PCR video, Simon Walsh (UK) interviews Pascal Meier (Switzerland) about the potential of social media to contribute to overall education and knowledge of interventional cardiologists, and to actually improve the clinical management of patients as it facilitates the exchange of knowledge. However, the use of social media is not without risk.
Abbott Vascular has announced it has entered into an agreement to purchase Tendyne Holdings (focused on developing minimally invasive mitral valve replacement therapies) and secured an option to purchase Cephea Valve Technologies (which is developing a catheter-based mitral valve replacement therapy).
The results showed no statistically significant treatment differences between patients treated with the Bioabsorbable Cardiac Matrix and patients treated with placebo for both the primary and the secondary endpoints.
In addition to allowing cardiologists to advance stents and guidewires with precision using digital controls, the system enables physicians to perform procedures while seated in a lead-lined interventional cockpit protected from radiation exposure.
Natec Medical has received 510k approval from US Food and Drug Administration (FDA) for its Filao NC PTCA balloon catheter, Natec's third device to obtain FDA approval.
Boehringer Ingelheim has announced that the first US patients have been enrolled in its international clinical trial-RE-DUAL PCI, which is evaluating the efficacy and safety of dabigatran in patients with non-valvular atrial fibrillation who have undergone percutaneous coronary intervention (PCI).
Continuum Clinical's ninth annual survey reveals that drug and device companies understand the value of real world studies, but continue to struggle with strategic, operational and organisational hurdles.
A new clinical trial to test how a high dose of stem cells delivered via a method called retrograde coronary sinus infusion affects end stage heart failure patients is showing promising results.
Micell Technologies has begun enrolling patients in DESSOLVE C: a prospective, single-blind, multicentre, randomised, controlled clinical trial to demonstrate the efficacy and safety of its MiStent SES sirolimus-eluting absorbable polymer coronary stent system.
COUNTER HF is a prospective, randomised, multicentre, controlled study evaluating the safety and efficacy of the C-Pulse system for the treatment of NYHA Class III and ambulatory Class IV heart failure.
The growth will be driven primarily by new product approvals and a paradigm shift in the clinical treatment of vascular diseases towards minimally-invasive procedures, says research and consulting firm GlobalData.
Hospira’s bivalirudin for injection is available in a single-dose flip-top vial, which matches the current branded offering available.
Boston Scientific has received the CE Mark and FDA clearance for its Safari2 pre-shaped guidewire-a new and enhanced version of the Safari guidewire-for introducing and placing interventional devices within the heart (including those used in transcatheter aortic valve implantation).
St Jude Medical and Thoratec have announced that the Boards of Directors of both companies have unanimously approved a definitive agreement under which St Jude Medical will acquire all of the outstanding shares of Thoratec for $63.50 per share in a cash transaction valued at approximately $3.4 billion, net of cash acquired.
A former technology executive of Google and Acxiom, Mui will be responsible for guiding the scalable development of HeartFlow's technology platform.
Contrary to the results of previous studies, according to a substudy of the EUROMAX trial, the transradial approach for coronary interventions is not associated with an improvement in clinical outcomes compared with the transfemoral approach.
The MVAD Pump is a heart pump that supports a wide range of flows to enable circulatory support for patients with advanced heart failure.
The China Recovery Study is designed to evaluate the performance of OrbusNeich’s Combo stent compared with the Nano stent (Lepu Medical Technology).
Boston Scientific has initiated a study to evaluate its fully resorbable scaffold technology. FAST (Fully absorbable scaffold feasibility study) is a prospective, single-arm study designed to assess the safety and performance of this next-generation scaffold.
Tim Kinnaird explores the risk of stent thrombosis with current technologies, how new technologies may be able to reduce this risk, and how OCT can help to assess this risk.
CardioSource World News talks with Hans Gustav HÌürsted Thyregod, about the NOTION study - the first all-comers trial to randomise low-risk patients to transcatheter aortic valve implantation (TAVI) or surgical aortic valve replacement.
Vital Images’ 510(k) FDA-cleared computed tomography (CT) myocardial perfusion application is debuting at the 10th Annual Scientific Meeting of the Society of Cardiovascular Computed Tomography (SCCT; 16-19 July, Las Vegas).
According to a new survey published in EuroIntervention, men state that the long working hours and the need to be on call that is associated with being an interventional cardiologist are the key reasons why so few women choose the subspecialty.
Distal Access has announced that it has sold the rights to its SPINR platform for peripheral, coronary, and endoscopic use to Merit Medical System's NRI Limited. The SPINR high-performance guidewire controller is designed to improve guidewire control, torque, and performance.
Aurora St Luke's Medical Center (Milwaukee, USA), a national leader in heart and vascular care, has become the first hospital in the world to use 4D ultrasound software, designed by GE Healthcare, to evaluate heart conditions.
Cardiovascular Systems has completed enrolment in COAST (Coronary orbital atherectomy system trial), which is designed to assess the safety and efficacy, as well as economic outcomes, of the company's new micro crown Orbital Atherectomy System (OAS).
Thoratec has announced that its HeartMate PHP (Percutaneous Heart Pump) has received CE mark approval, permitting sale in the EU and other international countries. The approval was based on data from the first 30 patients enrolled in the HeartMate PHP SHIELD I CE mark trial.
Edwards Lifesciences has announced that it has agreed to acquire CardiAQ Valve Technologies, a privately held company and developer of a transcatheter mitral valve implantation (TMVI) system.
Sorin has announced the global launch of its latest generation of heating and cooling system. The company reports that the FlexTherm-the newest addition to Sorin Heartlink System-is fully integrated with Sorin S5 industry-leading heart-lung machine.
A new study indicates that nearly one third of in-hospital deaths after PCI can be attributed to acute kidney injury. It also shows that preventing nine cases of acute kidney injury could potentially prevent one death.
The FDA has strengthened its existing label warning that non-aspirin nonsteroidal anti-inflammatory drugs (NSAIDs) increase the chance of myocardial infarction (or stroke). This follows, according to the agency, a comprehensive review of new safety information.
The Medicines Company has authorised, in an agreement with Sandoz, the distribution of an authorised generic of bivalirudin (Angiomax) for injection in the USA. The company states that the agreement will help to ensure that bivalirudin remains a high quality product in this market.
The FDA has announced it has approved Boston Scientific's Promus Element Plus and Promus Premier everolimus-eluting, platinum chromium coronary stent systems (monorail and over-the-wire) systems.
Admedus has announced positive long-term data from the CardioCel phase II clinical trial assessing the efficacy and safety of the company's proprietary bio-scaffold which has been implanted to repair congenital heart disease defects.
This ORSIF video tells the story of one of the world's most prominent cardiovascular surgeons, Edward Diethrich, and the career-altering health issues he has faced as a result of chronic, low-level exposure to ionising radiation through his work.
A retrospective analysis, published in Heart, indicates that early discharge after transfemoral TAVI in selected patients does not increase the risk of death at 30 days compared with later discharge.
A study indicates that surgical aortic valve replacement using the right anterior minithoracomy approach and a sutureless valve is associated with a trend towards better postoperative outcomes than those with TAVI
The implant begins a European study to evaluate outcomes and complications using less invasive surgical placement through hemisternotomy and left thoracotomy techniques.
Drug-eluting stents with biodegradable polymers have theoretical advantages over drug-eluting stents with permanent polymers-even second-generation drug-eluting stents.
Chee Tang Chin presents his group's paper on the Asian perspective of the DAPT trial published in AsiaIntervention and also asks Thomas Cuisset for his projection for DAPT therapy in Europe.
EHJQCCO is the eleventh European Society of Cardiology journal and focuses on the quality of care affecting cardiovascular outcomes at hospital, regional, national, and international level.
Patients are experiencing significant delays in access to approved cardiovascular devices due to bureaucratic inefficiencies, a Devices White Paper from the Cardiovascular Round Table (CRT) has found.
Miracor Medical Systems has appointed an international scientific advisory council consisting of leading clinicians to support the further clinical and technological progress of its PiCSO acute myocardial infarction impulse system.
The study is the first blinded, randomised clinical trial evaluating a mitral valve repair device in patients with functional mitral regurgitation.
At the International Society of Stem Cell Research Annual Conference (24-27 June, Stockholm, Sweden), the company also reported a 70% quality of life improvement measured by the MLHF questionnaire.
The PLATINUM DIVERSITY trial was initiated last year with the aim of evaluating the clinical outcomes of an everolimus-eluting stent (Promus Premier, Boston Scientific) in patient populations that have been traditionally under-served by clinical trials (eg. women and minorities).
Robin Mathews (Duke Clinical Research Institute, Duke University Medical Centre, Durham, USA) and others report, in Circulation: Cardiovascular Quality Outcomes, that tailored patient education may represent an opportunity to optimise patient adherence.
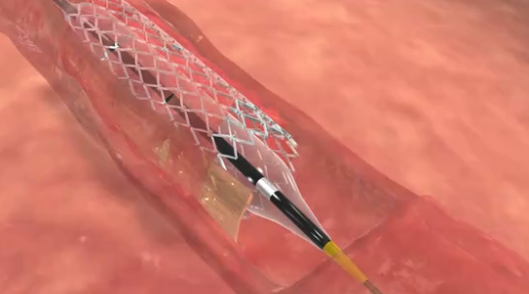
Medtronic has announced that the FDA has approved its recapturable, self-expanding CoreValve Evolut R TAVI system.
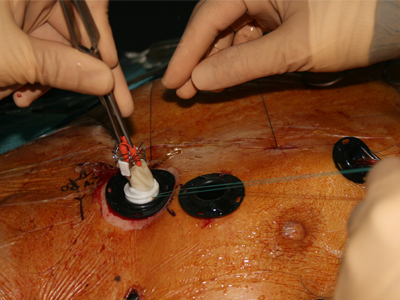
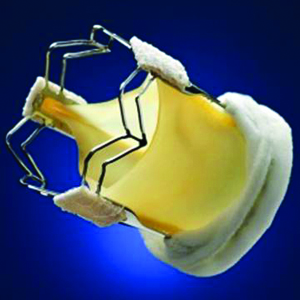
The second-generation bioprosthetic mitral heart valve was implanted into a 72-year-old male suffering from severe mitral regurgitation (MR 4+) with multiple co-morbidities and ineligible for alternate treatment modalities.
Jeffrey Cavendish, interventionalist and member of The Society for Cardiovascular Angiography and Interventions (SCAI) Foundation, and Donnette Smith, executive vice president of Mended Hearts, discuss the "After the Stent" campaign.
The application was based on the submission for US Food and Drug Administration (FDA) approval, which includes indications for use in cardiovascular repairs, suture buttressing and vascular and vessel repairs in both adults and children.
Delegates watched Arif Al Nooryani perform the triple-vessel disease case robotically in real-time by deploying bioresorbable stents, wiring the lesion and placing the stents using the CorPath system.
All three versions of the Turnpike are over-the-wire catheters with an advanced shaft design that provides superior tracking and advancement over a 0.014" guidewire for use in complex coronary and peripheral interventions.
The first US patient-Biotronik has revealed-has been enrolled in the BIOFLOW-V clinical study of the Orsiro hybrid drug-eluting stent. The aim of the study is further demonstrate the safety and effectiveness of the device.
Edwards Lifesciences has announced that the FDA has approved its latest transcatheter aortic valve implantation (TAVI) system-Sapien 3-for the treatment of high-risk patients suffering from severe, symptomatic aortic stenosis.
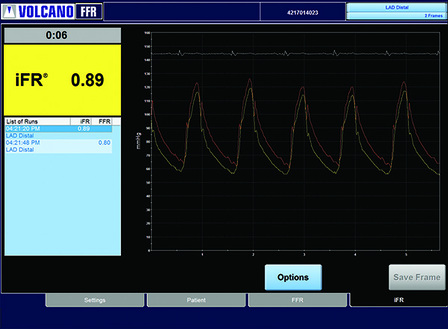
Opsens has received 510(k) clearance from the FDA for its OptoWire and OptoMonitor, its products, which have been developed to measure fractional flow reserve (FFR).
The call comes from the Cardiovascular Round Table, an independent forum established by the European Society of Cardiology and comprised of cardiologists and representatives of the pharmaceutical, device and equipment industries.
Poor sleep should be considered a risk factor for cardiovascular disease along with smoking, lack of exercise and poor diet.
Looking at images of their own calcified coronary arteries may be a wake-up call for patients with newly diagnosed coronary artery disease to change their lifestyles, reveals new research.
The tool was unveiled on the EPIQ 7 ultrasound system, Philips’ first ultrasound with anatomic intelligence capabilities, during the American Society of Echocardiography annual meeting (12-16 June, Boston, USA).
Following its 2012 survey of the barriers that prevent patients in Europe from having access to transcatheter aortic valve implantation (TAVI), BIBA MedTech has launched a new survey to gain insight into the barriers that stop patients outside of Europe from having this procedure.
The ACUITY study indicates that there are no significant differences in rates of myocardial infarction or death between diabetic patients with acute coronary syndromes and multivessel disease undergoing PCI and those undergoing CABG.
Amplicoat is a coating designed to enhance communication at the interface between human tissue and a medical device's electrode, and enable higher signal fidelity, reduced power requirements, and device electrode miniaturisation.
TCTAP 2015 Wrap-up Interview that explores the role of CABG vs. PCI for left main or three vessel disease. The moderator is David Paul Taggart with interviewees Seung-Jung Park and Patrick W. Serruys
Deepak L Bhatt (Executive director of Interventional Cardiovascular Programs, Brigham and Women's Hospital Heart & Vascular Center, Boston, USA) was the co-principal investigator of SYMPLICITY HTN-3, which he believes highlighted the value of sham-controlled trials.
The Melody transcatheter pulmonary valve (Medtronic), which received the CE mark in 2006 and FDA humanitarian device exemption approval in 2010, was recently granted FDA premarket approval. In this commentary, Darren P Berman explores the mid- and long-term data for the device
New results from two clinical studies, both of which were presented at EuroPCR (19-22 May, Paris, France) provide further support to the use of St Jude Medical's fractional flow reserve (FFR) technology to optimise percutaneous coronary intervention (PCI) procedures.
George joins co-principal investigator Murat Tuzcu, vice chairman of the department of cardiology for the Cleveland Clinic, in leading the trial.
Study finds significant tissue modification associated with orbital atherectomy led to better stent apposition and expansion.
Results in the first twelve months demonstrate rapid strut coverage, suggesting an early healing profile for patients using BioFreedom.
The FDA's expanded indications for use also allow the PleuraFlow system to be used in all cardiothoracic surgery and chest trauma procedures for adult and paediatric patients.
Albert Starr has received the 2015 Institut de France's Grand Prix Scientifique for work that led to the world's first successful artificial mitral valve implant. As a result of this success, Miles Lowell Edwards incorporated Edwards Laboratories to manufacture and market the Starr-Edwards valve
Carr-Brendel replaces Jan Keltjens, who served as interim chief executive officer since January 2015. Keltjens will remain chairman of the JenaValve board and will support Carr-Brendel during a transition period.
Merit Medical Systems has launched the Prelude Snap splittable sheath introducer, the design of which is based directly on physician feedback.
Philips has today announced the launch of "Ultrasound on Demand" in the UK, which it claims is the world's first on-demand ultrasound solution. The system aims to provide clinicians with access to the best technology at an affordable monthly fee, offering extensive functionality as well as meeting the ever-changing needs of busy hospital departments
The 7T Magnetom Terra is the first fully designed and manufactured by Siemens, with a new 7T magnet in its core, and comes with the lightest actively shielded 7T whole body magnet.
Toshiba says that the upgrades improve image quality and workflow "so those in both the clinical and research settings have access to the highest levels of performance and information to provide the best possible care."
European Bifurcation Club 10-year anniversary consensus document was the most downloaded EuroIntervention paper from the last 12 months. Lead Author Jens Flensted Lassen provides a brief summary of the main findings of this EBC document particularly in reference to the one vs two stent dilemma in coronary bifurcation treatment.
Daniel Simon reviews a new system that simulates fractional flow reserve (FFR) measurements using computed tomography (FFRCT, HeartFlow). He explains why he believes this system, which recently received FDA approval, has the potential to reduce the number of unnecessary coronary tests that are performed.
Results from the PRAGUE 13 trial indicate that there are no significant differences in outcomes between staged percutaneous coronary intervention (PCI) and culprit-only PCI in patients with multivessel disease; these results differ from those of earlier studies.
The clinical trial will examine the use of Thoratec's HeartMate PHP acute catheter-based heart pump in patients undergoing a high-risk percutaneous coronary intervention.
The retrospective study examined demographic, haemodynamic, and laboratory data in 26 consecutive subjects treated with a 50cc IABP and compared them with 26 patients receiving a 40cc IABP between 2012 and 2013.
The first edition of Current issues in PCI, which was published as a supplement ...
Results from the PARADIGM trial indicated that the CGuard system is appropriate for use in an all-comer carotid revascularisation population and is associated with favourable angiographic and clinical outcomes.
The Absorb GT1 combines a fully dissolving stent with a next-generation delivery catheter to help doctors treat people with heart disease.
Acute performance was demonstrated with 100% technical and procedural success and no reported major adverse cardiac events, with no incidence of ischaemic target lesion revascularisation, myocardial infarction or stent thrombosis.
The findings were announced at the Arterial Remodeling Technologies symposium at the EuroPCR 2015 meeting (19-22 May, Paris, France).
According to six-month data presented at EuroPCR (19-22 May, Paris, France), the Mitralign percutaneous annuloplasty system (MPAS) is associated with significantly improved valve function.
The final long-term results of the e-BioMatrix registry, which was presented at EuroPCR 2015 (19-22 May, Paris, France) by David Hildick-Smith (Sussex Cardiac Centre, Brighton, UK), have confirmed that the family of BioMatrix drug-eluting stents are safe and efficacious
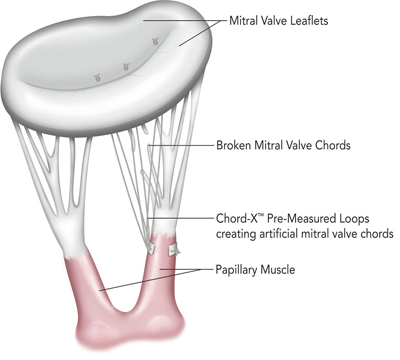
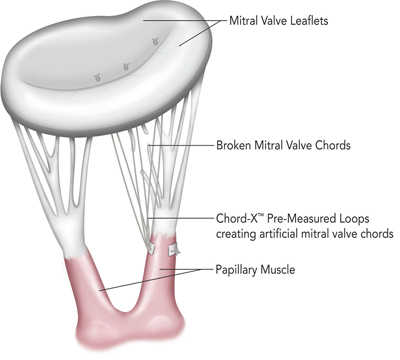
The On-X LTI Chord-X used during mitral valve repair is produced with On-X LTI’s proprietary ePTFE suture and has been designed to provide surgeons new tools to help simplify the procedure.
Stefan Verheye, co-principal investigator of the DESolve Nx trial, presented imaging results of multiple cases from a subset of 14 patients scheduled to be followed up with angiography and OCT at three years as part of the study.
Experts at the Biotronik-sponsored symposium discussed Orsiro's performance in difficult cases as well as coming developments in percutaneous coronary intervention.
A company press release states that the pause in enrolment is due to observed evidence of valve thrombosis that it believes warrants additional investigation.
The data were presented at EuroPCR 2015 (19-22 May, Paris, France) by Christoph C K Naber, from the Contilia Heart Centre in Essen, Germany.
The findings were presented at the EuroPCR 2015 conference (19-22 May, Paris, France) and were also published in the online edition of EuroIntervention.
Thirty-day results for the first 250 patients in the RESPOND post-market study were presented at EuroPCR 2015 by Nicolas M Van Mieghem, Erasmus Medical Center in Rotterdam, the Netherlands.
Results of the FFRCT RIPCORD study were presented at EuroPCR 2015 (19-22 May, Paris, France) by Nick Curzen of the University Hospital Southampton, UK.
The registration study will enrol 572 patients at up to 50 study locations in Japan and the USA.
These gender differences are reflected in the rate of risk factor control, which was lower in women, and in the rate of hospital readmission for a further heart attack, which was higher in women than in men.
Direct Flow Medical has announced positive two year data from the DISCOVER CE mark trial studying its Transcatheter Aortic Valve System at EuroPCR 2915 (19-22 May, Paris, France).
Presented as a late-breaking clinical trial at EuroPCR, new results from the NOTION trial showed that TAVI with CoreValve is as safe and effective as surgery in patients that are at a low- or intermediate-risk for surgery.
Additional data from EVOLVE II pivotal trial demonstrate safety and performance in patients with diabetes at one year.
Implantations in aortic stenosis patients at the University Heart Center Hamburg-Eppendorf (UKE), Germany, confirm safety at 30 days.
Trial designed to assess whether Adaptive Servo-Ventilation therapy could reduce mortality and morbidity in moderate to severe predominant central sleep apnoea patients with symptomatic chronic heart failure in addition to optimised medical care
Hospitals in the Midwest of the USA were more likely than others to refer patients for guideline-recommended cardiac rehabilitation following angioplasty, possibly because more rehab programs are available in the region.
The initial first-in-human procedures were performed at the Department of Cardiovascular Surgery and Transplantology, Jagiellonian University John Paul II Hospital, Poland.
The study examined the use of Cardiovascular Systems’ Diamondback 360 device in treating patients with de novo severely calcified coronary lesions.
With drug-coated balloons, we may have been able to deliver a device to the lesion, but we were not always sure how much drug we actually delivered. Fortunately, because of this problem, there have been huge improvements to drug-coated balloon technology. However, certain "tricks" are needed to achieve optimum results with drug-coated balloons.
The new release includes an optimised mitral workflow and a new septal crossing workflow for planning of mitral valve procedures to determine the appropriate access route based on computed tomography images.
Medtronic has announced the start of a clinical study using Medtronic technologies to determine whether paroxysmal and persistent atrial fibrillation can be treated with a combination of two ablation procedures targeting different anatomical locations.
ResMed has announced the launch of its ResMed Air Solutions range, led by the intelligent, cloud-enabled AirCurve 10 CS PaceWave with AirView system. This is the first device to combine remote monitoring with ResMed's iPaceWave minute ventilation (MV)-adaptive-servo-ventilation (ASV) therapy.
Boulle Medtech, a Jean Boulle Group medical technology company and the founding investor of Tendyne Holdings, has announced that the Tendyne transcatheter mitral valve implant was successfully implanted in the first patient in the USA.
Infraredx has announced the enrollment of 1,000 patients in its Lipid-Rich Plaque (LRP) study. The study is a prospective, multicentre clinical trial designed to identify a correlation between lipid-rich plaques detected by the company's TVC Imaging System and the occurrence of a cardiac event within two years.tvc
STENTYS will officially launch Xposition S at the EuroPCR conference on 19 May, 2015.
Boston Scientific has announced that it is taking a new approach to evaluate the performance of its Vessix renal Denervation System. It says it is initiating a study with a novel design to isolate the effects of the therapy in patients with high blood pressure.
The Medicines Company has announced that it has received the CE mark for cangrelor (Kangrexal) the first and only intravenous antiplatelet agent that provides immediate, consistent, and rapidly reversible P2Y12 inhibition.
An expert consensus statement released today by the Society for Cardiovascular Angiography and Interventions (SCAI), American College of Cardiology (ACC), Heart Failure Society of America (HFSA) and The Society of Thoracic Surgeons (STS) provides new guidance to help physicians match the right device with the right patient.
Tendyne has announced that its bioprosthetic mitral valve was successfully implanted in the first patient in the USA as part of a multicentre global feasibility study that aims to provide early insights into the safety and performance of the device.
CardiAQ Valve Technologies has received FDA investigational device exemption approval for a US early feasibility study of its second-generation transfemoral and transapical transcatheter mitral valve implantation system.
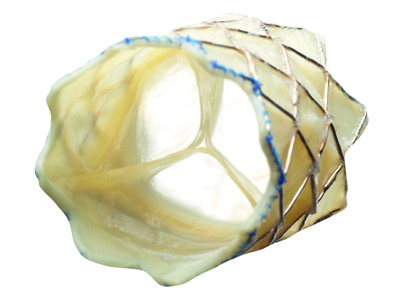
Patients with On-X aortic heart valves may be able to reduce their regular blood-thinning medication regimen, thanks to an expanded labelling claim granted by the US FDA to On-X Life Technologies.
Direct Flow Medical has received investigational device exemption approval from the US FDA to broaden its SALUS trial, including the addition of high risk patients and randomisation against Medtronic’s CoreValve.
Maquet Cardiovascular USA has announced publication of a study comparing the impact of percutaneous ventricular assist devices with intraaortic balloon pumps for high-risk patients undergoing percutaneous coronary intervention.
Ryan Madder, an interventional cardiologist at the Frederik Meijer Heart & Vascular Institute (Spectrum Health, Grand Rapids, Michigan, USA) explains why he believes a robotic system (CorPath, Corindus Vascular Robotics) for performing percutaneous coronary intervention (PCI) may help to reduce radiation exposure.
A meta-analysis of site-specific bleeding after percutaneous coronary intervention indicates that non-access site bleeding is associated with a significantly worse prognosis than is access-site bleeding.
The first safety and feasibility study of pressure-controlled intermittent coronary sinus occlusion (PICSO) in the setting of ST-segment elevation myocardial infarction indicates that the system could potentially enhance myocardial recovery after primary percutaneous coronary intervention.
A new study published in Stem Cells Translational Medicine demonstrates how mesenchymal stem cells not only protect the heart from further damage after a cardiac incident but can also slow down its ageing process.
The Organization for Occupational Radiation Safety in Interventional Fluoroscopy is a non-profit association raising awareness of health risks associated with fluoroscopy in catheterisation labs and radiographic diagnostic laboratories.
The workhorse balloon is designed to enhance surgical access to difficult lesions and challenging patient anatomies.
Admedus' CardioCel will be featured in a number of presentations at the American Association for Thoracic Surgery Mitral Conclave 2015 meeting and the 95th AATS Annual Meeting.
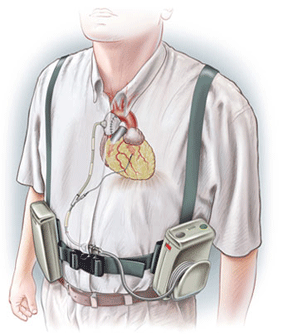
The FDA approval will allow SynCardia to launch the study with as many as 30 heart failure patients, who will receive the 50cc SynCardia Total Artificial Heart as a bridge to a donor heart transplant.
The FDA has approved the use of CoreValve for aortic valve-in-valve replacement procedures, meaning the transcatheter aortic valve implantation (TAVI) device is the first such device to be approved for valve-in-valve procedures both for patients who are at high risk and for those who are extreme risk for surgery in the USA
Cardiac anaesthesia investigators from Germany presented data at the International Anesthesia Research Society's (IARS) 2015 Annual Meeting and International Science Symposium.
Watchman offers a stroke risk reduction option for high-risk patients with non-valvular atrial fibrillation who are seeking an alternative to long-term warfarin therapy.
The transaction remains subject to conditions including approval by both Sorin and Cyberonics' shareholders, the receipt of required antitrust and regulatory clearances, and other customary closing conditions.
The new TC7PCI3212MT and TC7PCI3215MT support PCI Express Gen3 (8Gbps) and achieve wide bandwidth characteristics of 11.5GHz at -3dB.
Impella 2.5 is a ventricular support device used during high risk percutaneous coronary intervention in elective or urgent haemodynamically stable patients with severe coronary artery disease and depressed left ventricular ejection fraction.
DESSOLVE III is a randomised, controlled clinical trial comparing the MiStent sirolimus eluting absorbable polymer coronary stent system with the Xience everolimus eluting coronary stent system.
CardioKinetix has announced three-month clinical and echocardiographic results of PARACHUTE China, a study of 31 Chinese patients treated consecutively with the company's Parachute ventricular partitioning device.
Martin Cowie (Imperial College London, London, UK) is the principal investigator of the SERVE-HF study, which is assessing the use of adaptive servo-ventilation (PaceWave, ResMed) in chronic heart failure patients with central sleep apnoea. In this interview he explains why he believes the study, if positive, could convince cardiologist to start screening for the condition.
The results of the BEST study indicate that, in patients with multivessel disease, coronary artery bypass grafting (CABG) is associated with significant better outcomes than percutaneous coronary intervention (PCI) even in the era of second-generation drug-eluting stents. However, a registry study has indicated that there is no difference in mortality rates between PCI with everolimus-eluting stents and CABG
HeartIT has launched CloudCMR, designed to promote worldwide sharing of de-identified cardiovascular magnetic resonance images for education, research, and quality control purposes.
Toshiba showcased the Infinix 4DCT with the Aquilion PRIME CT at the American College of Cardiology annual meeting (14–16 March, San Diego, USA).
MitraClip is a treatment option for degenerative mitral regurgitation patients who are not good candidates for surgery the current standard of care because of their advanced age, frailty or other complicating factors.
The DEFLECT III trial indicates that use of the cerebral protection device TriGuard (Keystone Heart) in patients undergoing transcatheter aortic valve implantation (TAVI) is associated with an increase in the proportion of patients free from ischaemic brain lesions.
IntelliSpace Cardiovascular is a web-enabled image and information management system with an integrated workspace producing a holistic view of a patient’s care continuum across the entire cardiovascular service line.
Patients treated with the CardioMEMS heart failure system had a 57% reduction in mortality and a 43% reduction in heart failure hospitalisations compared with guideline directed medical therapy.
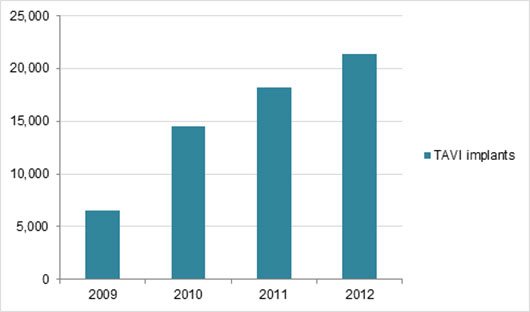
Data from 21,000 patient study was presented at American College of Cardiology 64th Annual Scientific Session and simultaneously published in the New England Journal of Medicine.
New platform enables controlled, polymer-free drug elution and is designed to help address next-generation technology challenges.
The researchers presenting the results at the American College of Cardiology 2015 meeting late-breaking clinical trial suggested that self-expanding TAVR should be considered as the new standard of care.
The device offers an alternative to long-term warfarin therapy for stroke risk reduction in patients with non-valvular atrial fibrillation.
Siemens offered a portfolio of systems and support solutions ranging from imaging modalities to information technology to in vitro diagnostics,.
Research supporting the value of adding NIRS-IVUS imaging to intravascular cardiology practice for detecting coronary artery disease will be presented throughout the ACC meeting.
Drug-eluting stent device with CoreWire Technology is to be studied in a wide spectrum of patients, including the smallest coronary arteries.
Ablative Solutions was the recipient of a "Top Cardiovascular Innovation Award" from Cardiovascular Research Technologies for the Peregrine system.
Mitralign transcatheter annuloplasty system could present a non-surgical option for both mitral and tricuspid valve regurgitation.
Admedus' CardioCel has entered the market in Hong Kong, continuing the expansion of CardioCel into Asian markets as part of Admedus' global product launch strategy.
US$15.2m investment from international investors rounded out financing. The funds will support a landmark trial and expand the company’s commercial operations.
CREDIT II is the first randomised controlled trial involving the Excel II coronary stent. Excel II is the latest generation in the Excel family of biodegradable polymer drug-eluting stents.
Initial clinical outcomes of Forge Medical's VasoStat haemostasis device were presented at the European Congress of Radiology (ECR) (4-8 March, Vienna, Austria).
This pause in enrolment is in accordance with the study protocol where in the event that more than three of the first twenty subjects pass away for any reason, the company will work with the FDA to discuss a plan to resume enrolment.
The EmpowerCTA+ has initially been launched in the USA and gradually in Europe, and was presented at the upcoming European Congress of Radiology (4–8 March, Vienna, Austria).
A new study indicates that while peritoneal lavage is a feasible method of achieving rapid cooling in patients with ST-segment elevation myocardial infarction (STEMI), it does not reduce infarct size and is associated with a significantly increased rate of adverse events.
A retrospective study indicates that drug-coated balloons are associated with similar angiographic and clinical outcomes to drug-eluting stents (including both first- and second-generation stents) for the management of in-stent restenosis.
Flavio Ribichini speaks to Cardiovascular News about being involved in the first use of primary angioplasty in Italy and how his belief in the "learning-teaching continuum" informs his clinical practice.
Prior to joining OrbusNeich, Aimonetti held a variety of senior roles in the cardio- and endovascular medical devices industry.
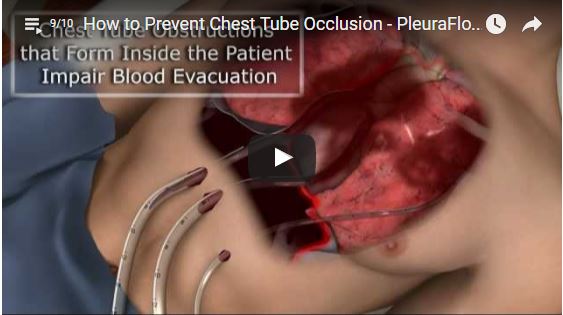
The PleuraFlow System uses a first-of-its-kind technology to enable caregivers to proactively keep chest drainage tubes clear of blood clotting after heart surgery.
Alvimedica has announced CE mark in Europe for the Cre8 46mm polymer-free Amphilimus eluting stent system, dedicated to long blockages in the vessels due to coronary artery disease.
The quantitative regurgitation workflow provides quantification of aortic regurgitation by using density of contrast in the aortic root and ventricle based on X-ray aortogram images.
ORBIT II is a study of the company's Diamondback 360 coronary orbital atherectomy system (OAS) in treating severely calcified lesions.
Completed enrolment was ahead of schedule and supports submission for FDA approval of the novel Cobra PzF stent with its advanced, nano-thin coating of Polyzene-F polymer.
DREAMS is an absorbable scaffold that combines the mechanical advantages of a metallic stent with a reliable bioabsorption profile that keeps the vessels open while avoiding the long-term disadvantages of permanent metal stents.
A new meta-analysis shows that bivalirudin significantly increases the risk of early stent thrombosis in patients undergoing percutaneous coronary intervention compared with other antithrombotic therapies. However, this increase in stent thrombosis is not associated with an increased risk of death or myocardial infarction.
A new study indicates that patients undergoing transcatheter aortic valve implantation (TAVI) who have moderate-to-severe chronic kidney disease have significantly worse outcomes than those undergoing TAVI who have mild disease.
Anson Cheung (University of British Columbia, St. Paul's Hospital, Vancouver, Canada) speaks to Cardiovascular News about the potential benefits of transcatheter mitral valve implantation.
Paul T Campbell has explored the stent savings made possible when robotic percutaneous coronary intervention (PCI) is leveraged for the measurement of anatomy in place of manual methods of measurement.
Seventy-four per cent of patients who were supported by the SynCardia temporary Total Artificial Heart for more than a year were either bridged to a donor heart or awaiting one.
The first patient case with the Euphora was recently performed by Richard Edwards, consultant cardiologist at the Freeman Hospital in Newcastle, UK.
Collaboration features latest Asahi technology in design, development and supply of core wire and coil assembly for the Svelte drug-eluting coronary stent fixed wire integrated delivery system.
Study showed superiority of Self-Apposing technology in opening the heart’s main artery.
London researchers to develop new MRI technique to improve diagnosis.
The HALO Trial represents a new opportunity for surgeons seeking a valve replacement for paediatric patients with no alternative approved treatment options.
Michael Weber is currently a professor of medicine at SUNY Downstate College of Medicine in, New York City, USA and the editor-in-chief of The Journal of Clinical Hypertension.
The LEADERS Free Japan is studying BioFreedom, the company's novel polymer and carrier-free drug-coated stent.
Approval allows MicroPort to commence commercialisation of Firehawk in the European market.
Novel drug-delivery technology provides sirolimus-eluting stent with unique bioabsorbable polymer absorption and drug-release profile.
The Stenting in Complex PCI meeting (2 December 2014, Milan, Italy) reviewed som...
The study is intended to support Japanese government approval of the Orsiro hybrid drug-eluting stent, which received CE mark in 2011.
The three studies were presented as late-breaking trials at the 2014 Transcatheter Cardiovascular Therapy (TCT) meeting (13–17 September, Washington, DC, USA).
Recently approved by the Food and Drug Administration, the MitraClip holds together the mitral valve's leaflets to reduce the degree of regurgitation caused by mitral valve regurgitation.
Guillaume Cayla explores how the duration of DAPT may be tailored after careful evaluation of both bleeding and ischaemic risk, taking into consideration the type of the device implanted.
CE mark approval received for new 26mm and 29mm valves.
Deborah A Quinn appointed vice president and medical lead for INOpulse programmes and Martin Dekker joins as vice president of device engineering.
The analysis was presented at the 2015 Annual Meeting of The Society of Thoracic Surgeons in San Diego, USA.
The agreement covers certain international markets, but excludes the USA and Japan.
He replaces David J Drachman, and will assume the role effective immediately and until a permanent replacement for Drachman has been hired.
The study is being conducted to support submission for FDA approval for the novel sutureless aortic valve.
The On-X LTI system for mitral valve chordal repair is produced with On-X LTI’s proprietary ePTFE suture and the company says it will provide surgeons new tools to help simplify the procedure.
The Final Appraisal Determination is the final phase in a multi-step review process by NICE and recognises the improved patient outcomes using Xarelto as a treatment option on top of dual antiplatelet therapy.
The funding will be used to support the launch of FEops' first product, TAVIguide a cloud-based pre-operative planning service for transcatheter aortic valve implantation in key markets in Europe and the USA.
Results showed that Abbott's test was able to diagnose a myocardial infarction in 22% of cases for women compared to a standard of 11%, when using a sex-specific threshold.
Results from a registry of 1,000 patients undergoing transcatheter aortic valve implantation (TAVI) in a high-volume centre do not indicate a significant difference in the rates of short- and long-term mortality between patients undergoing transapical TAVI and those undergoing transfemoral TAVI.
Justin E Davis, Imperial College, London, UK, presents a live case demonstration of the use of coronary physiology for the assessment of multi-vessel disease, using fractional flow reserve was to identify significant from non-significant coronary stenoses.
Rafael Bayer, co-chairman of the Innovation in Cardiovascular Intervention (ICI) meeting (14–16 December 2014, Tel Aviv, Israel), speaks to Cardiovascular News about the event's unique features.
The preferred provider collaboration will offer a set of clinical trial services to developers of cardiovascular drugs and devices.
A study of portable ultrasound carried out in the USA, Canada and India has revealed the potential of this technology for detecting plaques in peripheral arteries in both developed and developing country settings.
The secure transapical access and closure study(STASIS) is a non-randomised, multicentre, CE mark study, evaluating the safety and performance of Permaseal in transcatheter valve replacement procedures.
The pre-mounted, valved stent is folded without the need of an outer sheath, thereby enabling precision controlled positioning and also allowing for repositioning if needed.
BioGenerator, a minimally invasive nanotechnology solution, will treat the leading cause of US deaths.
Transcatheter Technologies GmbH has expanded the Trinity technology platform to include a transfemoral version.
The goal of CytoSorb treatment is to reduce inflammatory mediators and proteins such as cytokines and plasma free haemoglobin generated during surgery that can lead to serious post-operative complications.
Noemi C Espinosa brings to HeartFlow 30 years of experience as both a business executive and outside counsel, representing medical device, life science and high tech companies.
The Tendyne transcatheter mitral valve implant was successfully placed in the first patient enrolled in a three-continent, multicentre trial being conducted as part of the Tendyne feasibility study.
The American College of Cardiology and the American Heart Association have released clinical data standards for cardiovascular and endpoints in clinical trials.
Cardio3 BioSciences has announced the enrolment of the 240th patient in its CHART-1 European trial for C-Cure, the first and only stem cell therapeutic using guided stem cells for the treatment of congestive heart failure.
Henry Ford cardiologist Adam Greenbaum was asked to share the technique with Markus Kasel at the German Heart Centre Munich.
The Memo 3D ReChord incorporates a chordal guide system into the existing Memo 3D ring to simplify and standardise the approach to artificial chord replacement.
This decision represents CVRx's first commercial approval in the USA, based on an FDA determination that neo legacy is safe and can be used in US patients defined as responders to the Rheos Carotid Sinus Lead System.
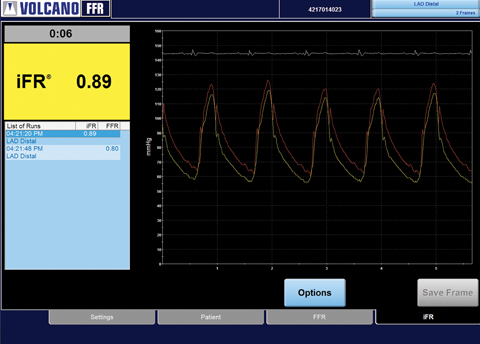
More than 1,000 systems have been activated with Volcano's instant wave-Free Ratio, allowing physicians and patients to benefit from a simplified workflow and a reduced need for hyperaemic agents.
CVRx has been granted CE marking to expand labelling of the Barostim neo system as safe for use in magnetic resonance imaging (MRI) systems under specified conditions.
Heart Research UK is accepting outline applications for its latest Novel and Emerging Technologies (NET) grant round worth up to £250,000.
HeartFlow has named former Johnson & Johnson chairman and chief executive officer William C Weldon as a new member of its board of directors.
Infraredx has announced results from an independent, prospective outcomes study evaluating the ability of intravascular near-infrared spectroscopy to identify lipid core-containing plaques.
Daniel O' Hair believes hybrid ORs may provide distinct advantages for complex TAVI cases. He speaks to Cardiovascular News about his experience of performing TAVI in a hybrid OR.
The Tendyne Transcatheter Mitral Valve system has been successfully implanted in three patients at the Royal Brompton Hospital in London, UK, under a compassionate use protocol.
The 4th edition of GulfPCR-GIM 2014, which takes place in Dubai, UAE, will bring together interventional cardiologists and cathlab staff from throughout the world to share experience and knowledge in order to improve cardiovascular care for all patients.
BIOTRONIK has enrolled the final patient in the BIOHELIX-I clinical trial to evaluate the safety and efficacy of the PRO-Kinetic Energy coronary bare metal stent.
REVA Medical has initiated patient enrollment with its Fantom bioresorbable drug-eluting scaffold.
In a new consensus statement, the Joint UK Societies claim that the negative results of SYMPLICITY HTN-3 should not be used as a "rationale for abandoning" renal denervation as a "novel therapeutic development" for resistant hypertension.
Neovasc has enrolled the first patient in the European arm of its TIARA-I early feasibility trial, a multinational, multicentre trial being conducted at centres in the USA, Europe and Canada.
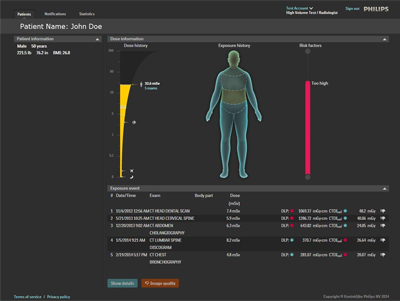
Royal Philips has introduced the DoseWise Portal, a comprehensive radiation dose management software solution aimed at managing radiation exposure risk to patients and their caregivers.
A team at Henry Ford Hospital, Detroit, USA, has successfully performed the first transcatheter tricuspid valve replacement surgery in the USA.
The combination of coronary artery bypass grafting (CABG) and mitral valve repair is not associated with additional benefits compared with CABG alone in patients with multivessel disease and moderate functional mitral regurgitation.
Toshiba America Medical Systems' Dose Tracking System (DTS) was awarded the Innovative Technology designation by Novation at its Innovative Technology Expo.
THE SCAI has responded to a study presented at the AHA Scientific Sessions examining the risks and benefits of continuing dual antiplatelet therapy beyond one year after placement of one or more drug-eluting stents as compared with aspirin therapy alone.
Innovative Cardiovascular Solutions has completed a Class A Unit financing totalling US$5m to fund its Emblok embolic protection catheter.
The US Food and Drug Administration has cleared the marketing of the HeartFlow FFR-CT software, which permits health care professionals to non-invasively evaluate blood flow in the coronary arteries of patients showing signs and symptoms of coronary artery disease.
Baylis Medical has announced the official opening of its new office in Watford, UK, on the outskirts of London.
Complete evacuation of blood from the pleural and pericardial spaces after cardiac surgery is critical. Clearflow explain that retained blood in these areas can cause acute and subacute complications (such as tamponade).
Qvanteq AG has enrolled the first patient in the First in Man clinical study QUEST I, following earlier regulatory approval from the Dutch and Swiss authorities.
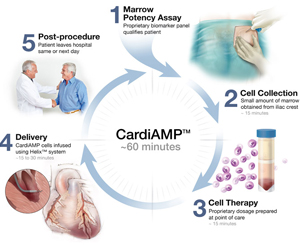
BioCardia has received permission to begin a phase III clinical trial of its bone marrow-derived CardiAMP therapy for heart failure after clearance from the US Food and Drug Administration (FDA).
In the first successful US pivotal trial of a bioabsorbable polymer stent, the Boston Scientific everolimus-eluting bioabsorbable polymer platinum chromium coronary stent system met its primary endpoint.
Shockwave Medical announced positive clinical results from the DISRUPT PAD study evaluating the safety and utility of Lithoplasty balloon catheters for the treatment of peripheral artery disease, at the Vascular Interventional Advances (VIVA) Annual Conference in Las Vegas, USA.
A multidisciplinary group of cardiac surgeons and interventional cardiologists, Heart Valve Voice, are calling for improved care of patients with structural heart disease in the UK, claiming that the country is "lagging behind" the rest of Europe in the number of aortic valve replacements and TAVI procedures it performs.
A 19-year-old woman was successfully implanted with the SynCardia temporary Total Artificial Heart and bridged to a donor heart transplant after cardiac surgeons used 3D virtual implantation to determine she was fit-eligible for the procedure, according to a case series in November's The Journal of Heart and Lung Transplantation.
Sanjit Jolly writes that data suggest that impaired microvascular perfusion after primary percutaneous coronary intervention (PCI) is strongly associated with subsequent mortality.1 Manual thrombectomy aims to reduce distal embolisation and improve microvascular perfusion during primary PCI.
A first-in-man study suggests that an injectable bioresorbable scaffold that is designed to prevent left ventricular remodelling after a large myocardial infarction is well tolerated and, therefore, could be a potential new therapeutic option for STEMI patients.
STENTYS has entered into a five-year agreement with Micell Technologies to be the exclusive distributor of the MiStent coronary stent worldwide (excluding the United States, Canada, China, South Korea and Japan).
Medtronic has undertaken the international launch of its Resolute Onyx drug-eluting stent following the receipt of CE mark approval. The first live patient implant of the Resolute Onyx occurred during the XII International Course of Endovascular and Myocardial Therapy in Madrid, Spain.
Essential Medical has received CE mark approval for its new vascular closure device, X-Seal. X-Seal closes femoral arterial punctures made during cardiac catheterisation procedures such as angiograms, angioplasty, and stenting.
The ESC has now announced that the world's largest cardiology meeting will be held in Barcelona in 2017 and Munich in 2018.
Medtronic has initiated the PERIGON (pericardial surgical aortic valve replacement) pivotal trial, a global, prospective clinical trial evaluating an investigational surgical aortic heart valve made from bovine pericardial tissue that is intended to replace a diseased, damaged or malfunctioning native or prosthetic aortic valve.
Stentys has announced it received CE marking for its sirolimus-eluting stent (SES). The CE marking will allow the company to market its SES in Europe immediately and, starting in 2015, in the other countries where the company has commercial activity.
The new HealthTap Concierge enables US doctors to connect privately with their own patients via HD video or secure text, and conduct paid consults at their convenience.
Cardio3 BioSciences, a company working on the discovery and development of regenerative, protective and reconstructive therapies, has announced the appointment of Warren Sherman as chief medical officer, effective as of 1 November 2014.
The TransMedics Organ Care System (OCS) heart technology was used to perform the world's first series of adult human heart transplants from donors after circulatory death (DCD donors) at St Vincent's Hospital in Sydney, Australia.
The TransMedics Organ Care System (OCS) heart technology was used to perform the world’s first series of adult human heart transplants from donors after circulatory death (DCD donors) at St Vincent’s Hospital in Sydney, Australia.
In an analysis that included approximately 35,000 participants, genetic predisposition to elevated low-density lipoprotein cholesterol (LDL-C) was associated with aortic valve calcium and narrowing of the aortic valve.
The National Institute for Health and Care Excellence (NICE) issued its appraisal consultation document (ACD) recommending Xarelto (rivaroxaban) 2.5mg twice daily as an option for secondary prevention in acute coronary syndromes.
The first implant was presented at the 28th Annual Meeting of the European Association for Cardiothoracic Surgery (EACTS) by Mattia Glauber.
The four-year mortality rate is significantly higher in patients with evidence of restenosis at routine control angiography than patients without evidence of restenosis. The rate is significantly increased even in patients with restenosis who are asymptomatic.
Sunil Rao speaks to Cardiovascular News about his career highlights, including his research into the transradial approach and exploring ways to improve the safety of antithrombotic drugs.
Neovasc has received FDA conditional approval to initiate its TIARA-I Trial in the USA. The
trial will evaluate the safety and performance of the company's Tiara mitral valve system.
Merit Medical has announced that it has launched a new website and educational initiative (thinkradial.com). The aim is to provide specialised training courses and information to help interventional cardiologists master the necessary skills.
Boston Scientific has initiated a new study of its second-generation everolimus-eluting coronary stent (Promus Premier) to evaluate its use in under-served patient populations, such as women and African Americans.
Admedus has announced that it now has a medical device licence to market CardioCel in Canada, which means that Canada becomes the latest market in the global launch of the product following sales in Europe and the USA.
Ablative Solutions has announced that the first patient in the Peregrine Study has been treated with company's the investigational Peregrine system, which delivers agents directly to the peri-adventitial area of the renal artery.
Three studies presented at the 2014 EACTS annual meeting showed positive data for Sorin's sutureless Perceval aortic valve.
Ziehm Imaging has been awarded with the Gold Stevie Award in the category "New Product & Product Management – Health and Pharmaceuticals" in the 11th annual International Business Awards.
A new study of men and women who were already being treated for heart disease, published in the Journal of the American College of Cardiology, shows that men and women have different cardiovascular and psychological reactions to mental stress.
Interim results from the PROACT (Prospective randomised On-X anticoagulation clinical trial) study indicate that patients with the On-X Plus 1.5 aortic heart valve may be safely managed at lower INR rates.
Data from a late-breaking trial (ADVANCE Direct Aortic) presented at EACTS indicate that the direct aortic approach with the CoreValve TAVI system is safe and effective for aortic stenosis patients at who are not suitable for the transfemoral approach.
ClearFlow has revealed positive results from its PRO-ACT (Prevention of retained blood outcomes using active clearance technology) study, which is evaluating its PleuraFlow Active clearance technology system to prevent retained blood complications after heart surgery.
Bracco Diagnostics has receives FDA approval for a new ultrasound contrast agent Lumason (sulfur hexafluoride lipid-type A microspheres) for injectable suspension, which is indicated for adults with suboptimal echocardiograms.
Admedus has announced its attendance at the European Association for Cardio-Thoracic Surgery (EACTS) 28th annual meeting (11–15 October, Milan, Italy) and will be showcasing CardioCel, its advanced cardiovascular scaffold, at the conference.
Biotronik has released Orsiro, a drug-euting stent that combines an active biolute coating and a passive probio coating, on to the French market. The release comes after a succession of studies demonstrating the safety and efficiency of the stent.
Materialise has listed its 3D-printed cardiovascular HeartPrint model as a medical device in the USA and EU markets. After years of 3D printing anatomical models for educational and research purposes, the company addressed the need for models that can assist with diagnosing, planning and practicing complex cardiovascular procedures.
Sorin Group has announced that the first US implant of Solo Smart was performed by David Heimansohn, St. Vincent's Heart Hospital, Indianapolis, USA.
At the 2014 Transcatheter Cardiovascular Therapeutics (TCT) meeting, Osprey Medical introduced its enhanced contrast-monitoring technology. The new Avert Plus system incorporates a new smart syringe and LCD display system with the existing Avert system.
The National Institute for Health and Care Excellence (NICE) has recommended Abbott's Architect Stat High Sensitive Troponin-I (hsTnl) test, among others, to help doctors quickly rule out heart attacks in NHS emergency departments in England and Wales.
CVRx has received CE mark approval of the Barostim neo system for the treatment of heart failure. The system is already CE-mark approved for the treatment of resistant hypertension.
The first patient in the randomised-controlled SENTINEL trial, which is evaluating the role of Sentinel cerebral protection device to reduce the risk of patients in patients undergoing transcatheter aortic valve implantation, has been treated.
AstraZeneca has announced that the American Heart Association/American College of Cardiology, in their updated the guideline for the management of patients with non–ST-elevation acute coronary syndromes (NSTE-ACS), has given Class IIa recommendation for the use of ticagrelor (Brilinta) over clopidogrel in patients with NSTE-ACS undergoing early invasive or ischaemia-guided strategy or receiving a stent.
CFI Medical has announced CE mark approval for its Zero-Gravity suspended radiation protection system floor unit, paving the way for its widespread use internationally. Biotronik will continue to act as the exclusive distributor of zero-gravity outside North America.
Direct Flow Medical has announced that it has received the CE mark for an enhanced delivery system for its TAVI valve. It says that the new delivery system allows for easy access and excellent trackability through calcified and tortuous anatomies.
Mitralign has reported on the successful use of its technology to perform a percutaneous repair on a patient with tricuspid regurgitation. The company also announced that Joachim Schofer and Rebecca Hahn presented details of the procedure at PCR London Valves.
Data presented at PCR London Valves (28–30 September, London, UK) indicate that the Acurate Neo transfemoral TAVI system, which recently received the CE mark, is not associated with any incidences of severe paravalvular leak.
Data from the US CoreValve High Risk study show that the incremental costs of TAVI with the CoreValve device in patients at high risk for surgery, compared with surgical aortic valve replacement, replacement are acceptable from a US perspective.
Azeem Latib (senior interventional cardiologist, San Raffaele Hospital and EMO-GVM Centro Cuore Columbus, Università Vita Salute, Milan, Italy) started his career in South Africa, relying on the hard work of his mother, numerous weekend jobs, and a community bursary to support his studies, but moved to Italy after meeting his "life-changing" mentor Antonio Colombo.
Boston Scientific has initiated the REPRISE III clinical trial, which a US study to evaluate the safety and effectiveness of the Lotus valve system in patients with severe aortic stenosis.
The PRIMA study shows that percutaneous closure of patent foramen ovale reduces migraine with aura days compared with medical therapy but does not reduce total migraine days
CardioKinetix has released results of a pooled analysis study of the first-of-its-kind catheter-based Parachute ventricular partitioning device.
Svelte Medical Systems reports that all endpoints in its DIRECT II study, which evaluated the company's drug-eluting coronary stent integrated delivery system, have been met. The study confirms results seen in prior studies in which the integrated delivery system demonstrated procedural time and cost savings.
Micell Technologies has announced that positive three-year clinical results from the DESSOLVE I and DESSOLVE II trials of its Mistent sirolimus-eluting stent with a biodegradable polymer were presented at the Transcatheter Cardiovascular Therapeutics (TCT) meeting (13–17 September, Washington, USA)
According to results from the APPOSITION III study, which was presented at the Transcatheter Cardiovascular Therapeutics meeting (13–17 September, Washington, USA), the Stentys' Self-Apposing stent is associated with a low rate of mortality at two years after a myocardial infarction.
Data presented at the Transcatheter Cardiovascular Therapeutics (TCT) meeting (13–17 September, Washington, USA) provided further evidence to support the healing benefits of the Combo dual therapy stent, which is the only drug eluting stent with active EPC capture technology.
Acist Medical Systems showcased the first-ever high-definition intravascular ultrasound (HDi) system during two live cases at the Transcatheter Cardiovascular Therapeutics meeting.
Cardiac Dimensions has announced that new long-term outcomes data from the TITAN II clinical trial of its enhanced Carillon mitral contour system showed significant and sustained improvements in mitral regurgitation, functional improvement, quality of life and reverse cardiac remodelling.
New data indicate that Boston Scientific's TAVI device, Lotus, is associated with sustained safety and performance at one year with no cases of moderate or severe paravalvular aortic regurgitation.
Symetis has announced that it has received the CE mark for its Acurate neo transfemoral system, which means it now offers (like market leaders Medtronic and Edwards Lifesciences) both transapical and transfemoral options for TAVI delivery.
CeloNova BioSciences has announced that positive first-in-man clinical trial results indicate that its Cobra PzF coronary stent system, which has a nano-thin coating of polyzene-F polymer, is a safe and effective routine treatment for real-world and complex patients with heart disease.
At one year, overall clinical outcomes for Absorb (Abbott) were comparable to Xience (Abbott), and people treated with Absorb experienced a significantly lower rate of angina.
Five-year clinical outcomes for inoperable patients treated in the PARTNER trial were presented as part of the late-breaking clinical trials session at the Transcatheter Cardiovascular Therapeutics (TCT) conference.
0
Medtronic has announced the US Food and Drug Administration (FDA) 510(k) clearance and launch of the NC Euphora noncompliant balloon dilatation catheter.
Micro Interventional Devices has reported the first successful clinical case using its Permaseal Cardiac Access and Closure technology. This new technology simplifies minimally invasive aortic valve replacement procedures.
Medtronic has announced the first US implants in the CoreValve Evolut R Clinical Study, which will evaluate the safety and effectiveness of the new Medtronic CoreValve Evolut R system.
In her PhD study, Tuija Kangasmaa, invented a method which makes it possible to reduce the imaging time by up to 50%, making the scan session easier for the patient.
The CE mark of the Enabler-C coronary catheter system follows the successful first-in-man study of the Enabler-C that took place at the Institut Cardiovasculaire Paris-Sud.
Robert Byrne writes that although drug-coated balloon therapy has been a significant innovation with relevance for everyday practice, convincing data in the coronary arena exists thus far only for the treatment of in-stent restenosis.
Stentys has announced that its Self-Apposing stent has been implanted more than 10,000 times in patients worldwide. A company press release reports that this milestone further illustrates the popularity of Stentys' technology among cardiologists in Europe and a growing number of regions globally.
Physicians at the Heart Hospital of Austin became the first in Texas to implant the new Portico re-sheathable transcatheter aortic valve implantation (TAVI) device as part of the PORTICO trial, which is a nationwide clinical study to examine the effectiveness of the new heart valve.
Biosensors has announced enrolment of the initial patient in BioFreedom USA, an investigational device exemption (IDE) feasibility trial designed to collect additional US-based safety and effectiveness data for BioFreedom, the company's novel polymer and carrier-free drug-coated stent.
Corindus Vascular Robotic will host the breakfast symposium "Robotic PCI: Precision and protection from occupational hazards" at the upcoming Transcatheter Cardiovascular Therapeutics (TCT; 13–17 September, Washington, USA).
Medtronic has announced that it has received the CE mark for its 23mm CoreValve Evolut R system for transcatheter aortic valve implantation (TAVI). A company press release states that the novel, self-expanding valve and 14FR equivalent delivery system offers new capabilities that advance valve performance and deliverability during the procedure.
The MITOCARE study indicates that the novel agent TRO40303 does not provide any protective effect compared with placebo in preventing reperfusion injury in STEMI patients undergoing PCI. This study, combined with many failures in the field, has raised questions about whether or not reperfusion injury actually occurs in man.
Pre-hospital administration of ticagrelor does not improve coronary reperfusion before percutaneous coronary intervention patients with ST-segment elevation myocardial infarction. However, it may reduce the risk of stent thrombosis.
Proceeds will fund randomised, pivotal SENTINEL trial to study how cerebral protection may reduce the incidence of stroke in transcatheter aortic valve implantation (TAVI).
Royal Philips has announced that it has received 510(k) clearance from the US Food and Drug Administration to market its precision planning application for transcatheter aortic-valve implantation (TAVI) treatments.
Direct Flow Medical has received the CE mark for a 23mm sized valve as part of its transcatheter aortic valve system. The company has also received the CE mark for implantation of all of its valves without the use of contrast media, protecting patients from kidney injury during transcatheter aortic valve implantation.
William H Crowder believes that, despite some US interventional cardiologists' reluctance to use the approach, the transradial approach will become standard of care for coronary catheterisation procedures.
A new study indicates the obesity paradox, in which being obese seems to confer a mortality benefit, observed in some studies of patients with ST-segment elevation myocardial infarction (STEMI) can be at least partly explained by confounders.
A network meta-analysis indicates that while drug-eluting balloons and drug-eluting stents are effective treatment options for drug-eluting stent restenosis, balloon angioplasty (by comparison) is not an effective treatment and should not be used.
Efforts over the past decade to improve the quality of care for cardiovascular disease patients and increase the use of evidence-based treatments have led to a significant drop in the rate of hospitalisations and deaths, according to a new study in Circulation.
A new study shows a strong association between severe, untreated obstructive sleep apnoea and the risk of elevated blood pressure despite the use of high blood pressure medications.
The study will evaluate the efficacy and safety of the oral anticoagulant dabigatran etexilate in patients with non-valvular atrial fibrillation, who have undergone a percutaneous coronary intervention (PCI) with stent placement to widen their blocked coronary arteries.
AstraZeneca has announced that it has received confirmation from the United States Department of Justice that it is closing its investigation into PLATO, a clinical trial with Brilinta (ticagrelor).
OrbusNeich has announced the appointment of B Wayne Johnson as president and chief executive officer, with responsibility for all commercial and clinical aspects of the company.
The two companies have reached an agreement to develop a new and differentiated FFR guidewire and next generation Boston Scientific Rotablator RotaWire.
Next year SERVE-HF, the world's largest randomised trial ever conducted to assess the benefits of effectively treating heart failure patients with sleep-disordered breathing, will be reporting results - setting the scene for major changes in how cardiologists view and manage this prevalent co-morbidity.
Cardium Therapeutics has announced the publication of a review article in the Journal of Cardiovascular Pharmacology that concludes a gene therapy product promoting the growth of blood vessels is "highly warranted" to treat heart-disease patients who are either ineligible or poor candidates for traditional angioplasty, stent placement or bypass surgery.
Juventas Therapeutics has announced that it has successfully completed the phase I arm of the RETRO-HF clinical trial, and fully enrolled the phase II arm that is evaluating the safety and preliminary clinical efficacy for retrograde infusion of JVS-100 in patients with heart failure.
Toshiba has announced that it will feature SURESubtraction Coronary at The European Society of Cardiology (ESC) congress (30 August–3 September, Barcelona, Spain).
JenaValve Technology has announced that cardiovascular medical device industry veteran Stefan Schreck has been named the company's new chief technology officer, effective immediately.
The Centers for Medicare & Medicaid Services recently issued a National Coverage Determination that extends coverage for Medicare beneficiaries in the United States to Transcatheter Mitral Valve Repair with Abbott's MitraClip system.
The paper compared the outcomes of 40 patients that underwent PCI procedures at a single site in the PRECISE trial using the CorPath robotic system to the 80 consecutive patients who met the same inclusion criteria but underwent conventional, manual PCIs at the same centre.
A study published ahead of print in the Journal of American Cardiology: Cardiovascular Interventions supports a minimalist transfemoral approach to transcatheter aortic valve implantation (TAVI) for the treatment of high-risk and inoperable patients with aortic stenosis.
Thoratec Corporation has announced that its CE mark clinical trial for HeartMate percutaneous heart pump (PHP) has commenced.
HeartWare International has announced the appointment of Katrin Leadley as chief medical officer effective 1 September, 2014.
James Cockburn, David Hildick-Smith, and Adam de Belder describe their recent study (published in the International Journal of Cardiology), which found that patients who underwent rotational atherectomy had a lower mid-term survival compared with patients who underwent conventional percutaneous coronary intervention (PCI).
The decision to place the trials on hold was based on a safety review of reported cerebrovascular events. Symptoms occurred in three patients, of which two patients' symptoms fully resolved within a short period of time and the third patient has had substantial resolution of symptoms.
According to a new study, a simple cost-effective multicentre quality improvement intervention could prevent contrast-induced acute kidney injury in one in five patients undergoing non-emergent percutaneous coronary intervention.
The OK gives the company control over the two critical components for heart manufacturing: The valves and the unique formula for segmented polyurethane solution, which is manufactured only by SynCardia.
ACIST Medical Systems has announced that it has entered into a strategic agreement with Medtronic to co-promote the world's first Rapid Exchange FFR (RXi) and High Definition IVUS (HDi) technologies in the United States.
An expert in cardiovascular imaging with a unique background in space medicine, Thomas will begin seeing patients at Northwestern Memorial Hospital in August. He is also a professor of medicine at Northwestern University Feinberg School of Medicine.
Solo Smart from Sorin Group is the first aortic valve with a removable stent, providing native-like performance with the ease of implant of a stented valve.
Tryton Medical has announced that the first patient has been enrolled in the EXTENDED Access Registry (Tryton IDE XA registry), a single arm study of its Tryton Side Branch stent.
Sorin Group has announced that it has been granted CE mark certification for its innovative stented aortic bioprosthesis Crown PRT (Phospholipid Reduction Treatment), now commercially available in Europe.
According to Boston Scientific, the Agent drug-coated balloon provides physicians with an additional alternative to treat both in-stent restenosis and de novo small vessel coronary disease.
The new PICSO (Pressure-controlled intermittent coronary sinus occlusion) impulse system, consisting of the impulse console and impulse balloon catheters, is CE-marked and has already been used to treat patients in the UK, Ireland, and Hungary.
Revised guidance from NICE, recommends once-daily, oral antiplatelet prasugrel (Efient), in combination with aspirin, as a cost-effective option for a wider group of acute coronary syndrome patients having primary or delayed PCI.
The Cardiovascular Cell Therapy Research Network has selected Cytori Therapeutics to supply adipose-derived regenerative cells for a clinical trial aimed at evaluating the safety and feasibility of treating patients with left ventricular assist devices.
Transcatheter Technologies has announced that results of a first-in-human clinical study of its Trinity system have been published ahead of print publication in the July issue of EuroIntervention.
According to Boston Scientific, the Polaris imaging system offers enhanced ease-of-use and more powerful processing capabilities.
The Rebel stent offers the identical stent platform as the Promus Premier drug-eluting stent but without the everolimus drug.
CorMatrix Cardiovascular has announced that it has received US Food and Drug Administration (FDA) clearance to market the CorMatrix ECM (extracellular matrix) for vascular repair.
BIBA MedTech announces the release of the Tavimonitor.org website application for computer desktops and Windows Phone 8 devices.
A senior researcher in biomedical ethics (David Shaw, Institute of Biomedical Ethics, University of Basel, Basel, Switzerland) has provided a robust defence of the controversial use of delayed consent in the HEAT-PPCI study, calling it "entirely ethical"
A meta-analysis supports previous findings that preoperative moderate-to-severe mitral regurgitation negatively affects outcomes after transcatheter aortic valve implantation (TAVI), but also indicated that there is a trend towards mitral regurgitation improvement after TAVI.
OrbusNeich has announced that the first patient has been enrolled in the Multinational abluminal sirolimus coated bio-engineered stent (MASCOT) post-marketing registry designed to assess the long-term safety and effectiveness of the Combo stent.
Through a percutaneous procedure now available through a clinical trial at PinnacleHealth CardioVascular Institute the first minimally invasive catheter-based device aims to restore normal geometry and function in the damaged left ventricle.
InspireMD has announced the appointment of James Barry as chief operating officer. Barry, who has more than two decades of experience in the medical device industry, will be based in the company's Boston, USA headquarters.
According to Paragonix, the Sherpa Perfusion cardiac transport system can be used for either hypothermic oxygenated perfusion or static storage of donor hearts during transport.
Boston Scientific Corporation has received CE mark approval and begun the European commercial launch of its new 25mm Lotus transcatheter aortic valve implantation (TAVI) system, complementing the currently available 23mm and 27mm valve sizes.
Infraredx has announced the first patient enrolled in PROSPECT II, a multicentre, prospective study designed to assess the ability of intravascular imaging to identify non-flow obstructing vulnerable plaques.
According to Toshiba, the Infinix Essential is an ideal value system that does not sacrifice performance. With its slim, off-centre C-arm design, the system allows steep angulations for optimised vessel profiling during cardiac interventions.
The FDA has informed Regado that a clinical hold has been placed on all patient enrolment and dosing of either study drug in the ongoing phase 3 REGULATE-PCI trial.
The phase II funding from the National Heart, Lung, and Blood Institute (NHLBI) is to continue the development of a bioresorbable, self-expanding scaffold to treat paediatric pulmonary artery stenosis.
STENTYS has established distributor agreements in Argentina, Chile and Colombia.
Thoratec Corporation has announced that it has acquired Apica Cardiovascular Limited for an upfront cash payment of US$35 million and potential future clinical and sales milestones of up to US$40 million.
CardiAQ recently filed a Lawsuit against Neovasc in Germany alleging that Neovasc's pending European patent application is based upon CardiAQ technology that Neovasc obtained from CardiAQ in 2009 and 2010 during a confidential supplier relationship.
JenaValve Technology has announced that medical device industry veteran David J Drachman has been named the company's new chief executive officer, effective immediately.
According to a report in The Journal of Thoracic and Cardiovascular Surgery, totally endoscopic aortic valve replacement (TEAVR) is technically feasible. Lead author of the report Marco Vola talks to Cardiovascular News about TEAVR and its potential role in the management of patients with aortic stenosis.
A study indicates that patients being assessed for possible transcatheter aortic valve implantation (TAVI) who have incidental findings on computed tomography aortogram are significantly less likely to receive TAVI as definitive therapy and a have worse overall come than patients without these findings.
A new study suggests that transradial access in patients with non-ST-segment elevation myocardial infarction (NSTEMI) undergoing percutaneous coronary intervention is associated with reduced bleeding and reduced mortality compared with transfemoral access.
CorMatrix Cardiovascular has announced the successful enrolment and treatment of five patients in the first ever study evaluating the CorMatrix ECM delivered trans-epicardially for the improvement of myocardial function in patients at risk or suffering from congestive heart failure.
The first cases using the ProTrack Pigtail wire in Europe were performed last month at St George's Hospital in London, UK. The ProTrack Pigtail wire is used during the transseptal procedure, a challenging minimally invasive technique used to cross the septum of the heart.
The Spectranetics Corporation has announced that it has completed the acquisition of AngioScore for US$230 million in cash, along with additional contingent commercial and regulatory milestone payments.
Corindus's CorPath vascular robotic system is the first medical device to bring robotic precision and accuracy to coronary angioplasty procedures, which may improve clinical outcomes while increasing radiation protection for interventional cardiologists.
Royal Philips and salesforce.com have announced a strategic alliance to deliver an open, cloud-based healthcare platform. The collaboration has already resulted in two clinical applications to be launched on the new platform later this summer: "Philips eCareCoordinator" and "Philips eCareCompanion."
Siemens Healthcare recently hosted a series of echocardiography and workflow efficiency workshops across the UK. Using live demonstrations of the ACUSON SC2000, delegates were able to discover how continuous volume imaging can improve diagnostic confidence and workflow efficiency.
The simulated procedure provides training in the evaluation of native valve diseases, prosthetic valve dysfunction, congenital or acquired heart diseases such as atrial fibrillations, coronary thromboses, coronary stenoses and more.
Doctors at Fortis Hospitals, Bangalore, India, performed minimally-invasive trans-abdominal coronary artery bypass graft (CABG). The procedure was performed by making only a small incision in the upper abdomen, sparing the breast bone or the chest wall completely.
According to results presented at EuroPCR (20–23 May, Paris, France), a self-apposing sirolimus-eluting stent is associated significantly faster full strut coverage and better apposition at four months than a balloon-expandable zotarolimus-eluting stent.
A novel anticoagulation system, comprising of a single-stranded RNA factor IXa inhibitor (pegnivacogin) and its reversal agent (anivamersen), was shown to be safe and feasible for supressing both ischaemic events and thrombotic complications with a favourable bleeding profile in patients with acute coronary syndromes undergoing percutaneous coronary intervention.
HLT has announced the addition of Kevin Bassett as general manager. In his new role, Bassett will lead the development and achievement of HLT's strategic business objectives and milestones in collaboration with parent company, Bracco Group.
Biosensors International has announced enrolment of the first patient in LEADERS Free Japan, a trial involving BioFreedom, the company's novel polymer and carrier-free drug-coated stent.
Edwards Lifesciences has announced that it has received US Food and Drug Administration (FDA) approval for its Sapien XT transcatheter aortic heart valve for the treatment of high-risk and inoperable patients suffering from severe symptomatic aortic stenosis.
FDA approval is based on research that showed clinical outcomes at one year with the CoreValve system (Medtronic) were superior to open heart surgery, the current gold standard for aortic valve replacement.
The principal investigators of the REDUCE (Randomised evaluation of short-term dual antiplatelet therapy in patients with acute coronary syndrome treated with the Combo dual-therapy stent) trial have announced the enrolment of the first patient at Isala Hospital, Zwolle, the Netherlands.
Iain A Simpson, president of the British Cardiovascular Society, highlights that the letter sent to NICE regarding their draft lipid guidance, by a number of healthcare professionals, including the President of the Royal College of Physicians of London, does not represent the views of the cardiovascular healthcare professionals in the UK.
The US Food and Drug Administration (FDA) has cleared the Chocolate percutaneous transluminal coronary angioplasty balloon catheter (Chocolate PTCA), for balloon dilatation of the stenotic portion of coronary artery or bypass graft stenosis for the purpose of improving myocardial perfusion.
One-year data from ORBIT II show strong rates of freedom from target revascularisation, cardiac death and major adverse cardiac events (MACE).
Stentys has announced the signing of the acquisition agreement of Cappella Peel Away Inc. (Delaware, USA) and its assets relating to a novel stent delivery system. The catheter technology will enable the implantation of the self-apposing stent in the same manner as a conventional balloon-expandable stent.
According to the 30-day outcomes of the Sapien 3 study, the Sapien 3 transcatheter heart valve is associated with a low mortality rate and a low stroke rate in transfemoral patients with aortic stenosis who are at high or intermediate surgical risk.
Data from the EXAMINATION trial indicates that manual thrombus aspiration prior to primary percutaneous coronary intervention (PCI) is associated with a higher rate of direct stenting and a lower rate of postdilation than no manual thrombus aspiration prior to primary PCI.
The Women Committee of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) has been set up to attain gender equality in interventional cardiology at the professional and patient level. The chair of the committee, Julinda Mehilli speaks to Cardiovascular News about why the comparative lack of women in interventional cardiology means that the specialty is "losing more than 50% of the talent pool".
Boston Scientific has initiated the RESPOND post market registry to assess real world performance of the Lotus valve system. The RESPOND registry will collect data on clinical outcomes and device performance in 1,000 patients implanted at 50 centres around the world.
After decades of researching why people with Mediterranean diets suffer fewer heart attacks and strokes, clinical researchers have pinpointed how lycopene, found in the skin of tomatoes, could reduce risks.
David J Cohen, director, Cardiovascular Research, Saint Luke's Mid America Heart Institute, Kansas City, USA, has conducted extensive research into the cost-effectiveness of interventional cardiology devices. He talks to Cardiovascular News about his career and why understanding the cost-effectiveness of devices is essential for the future of interventional cardiology.
Combo Dual Therapy stent has been shown to actively promote rapid endothelialisation and deliver long-term, true vessel healing.
Biosensors International Group has announced that Jose ("Pepe") Calle Gordo has been appointed the company's new chief executive officer, with effect from 1 November 2014.
With enrolment now complete, the baseline patient population data from Biosensors' LEADERS FREE study was presented for the first time at EuroPCR 2014 (20–23 May; Paris, France) by principal investigator Philip Urban.
Medtronic has announced CE mark approval and launch of the NC Euphora noncompliant balloon dilatation catheter. The NC Euphora balloon catheter is now available in Europe and other countries outside of the United States that recognise the CE mark. It is not yet available in the United States.
St Jude Medical has announced preliminary results from the EnligHTN III study, which found the company's second-generation EnligHTN renal denervation system provided safe and effective therapy for patients with drug-resistant, uncontrolled hypertension six months post-procedure.
Biotronik announced new results from the subgroup analyses of the BIOFLOW-II clinical trial at the EuroPCR 2014 congress in Paris, France. The results were presented by investigator Manel Sabaté, Hospital ClÃnico y Provincial de Barcelona, Barcelona, Spain.
According to the results of REPRISE II, only 1.1% of patients treated with the Lotus TAVI device (Boston Scientific) experienced moderate paravalvular aortic regurgitation with no severe cases of aortic regurgitation reported.
Boston Scientific reports positive three-year follow-up data for the EVOLVE clinical trial, comparing the safety and performance of the Synergy everolimus-eluting bioabsorbable polymer platinum chromium coronary stent system to the Promus element stent system.
The CE mark gives SynCardia systems control over the last critical component for SynCardia heart manufacturing.
InspireMD to present at European symposium titled: 'MGuard Embolic Protection Stent: The Importance of Thrombus Management in STEMI Primary PCI'
Holger Hotz (Charite University Medicine Berlin, Germany) writes about a novel, less invasive therapeutic option that has been developed to stop the progression of heart failure and to give patients the chance of recovery.
The results of its trial on the Freedom Solo (Sorin) for approval on the US market were presented at the 94th Annual Meeting of American Association for Thoracic Surgery (AATS) in Toronto, Canada.
The world's first scaffold with a dramatically thinner strut profile of 100µm (one hundred microns) is designed to degrade within one year, returning the patients' coronary vessel ultimately to its normal de novo state while providing market-leading deliverability and conformability.
St Jude Medical has announced the first patient implants of the Portico re-sheathable transcatheter aortic valve system occurred in US IDE trial (PORTICO trial).
Fortis has set up a world-class comprehensive centre for heart failure and heart transplant, at the Fortis Malar Hospital, in Chennai, India. The speciality centre under the leadership of K R Balakrishnan, director Cardiac Sciences and Suresh Rao K G, chief of Cardiac Anaesthesia and Critical Care, is ably supported by an experienced team of leading cardiac experts.
InspireMD has announced new results from the iMOS (International MGuard Observational Study) Prime registry demonstrating that use of the MGuard Prime in cases of acute ST-segment elevation myocardial infarction (STEMI) patients undergoing percutaneous coronary intervention resulted in complete ST-resolution in approximately 75% of cases, and a 2.2% rate of major adverse cardiac events at 30 days, including zero cases of mortality.
Distinguished physicians from leading cardiac surgery centres gathered at an educational symposium to share their experiences with a new-generation blood evacuation system for use following cardiothoracic surgical procedures.
The UK's leading children's heart charity is delighted that all babies in the UK are set to receive a life-saving test to detect heart conditions at birth.
Ablative Solutions has announced that it has added Paul Sobotka, a highly respected clinician, to its Scientific Advisory Board.
GE Healthcare has announced that it has received US Food and Drug Administration (FDA) clearance for its Discovery IGS 740, a new rail-free mobile angiography system with a 41x41cm detector. The Discovery IGS 740 is the first of this kind of mobile angiography system in the industry to receive FDA approval.
Biosensors has announced that the US Food and Drug Administration (FDA) recently granted conditional investigational device exemption (IDE) approval for a US-based clinical trial of the BioFreedom polymer-free drug-coated stent system.
The catheter is specifically designed for enhanced fast and accurate thrombus aspiration. The new device is being marketed in over 50 countries worldwide.
Medtronic has revealed new data demonstrating positive outcomes for extreme risk patients with severe aortic stenosis who were treated with the CoreValve system via alternative access approaches.
Stentys has announced that the Journal of the American College of Cardiology (JACC) published a "State-of-the-Art Paper" entitled "Mechanisms, Pathophysiology, and Clinical Aspects of Incomplete Stent Apposition," which highlights Stentys' self-apposing stent among novel devices to minimise stent malapposition in clinical practice.
Medtronic has announced five-year follow-up data demonstrating the safety and performance of the 3f Enable aortic bioprosthesis - the world's first commercially available sutureless tissue heart valve.
Boston Scientific Corporation has launched the Promus Premier everolimus-eluting platinum chromium coronary stent system in Japan. This advanced drug-eluting system recently received Japan Ministry of Health, Labour and Welfare approval.
Sorin Group has announced that it received US Food and Drug Administration (FDA) approval for the Mitroflow aortic pericardial heart valve with phospholipid reduction treatment.
Cardiosonic has announced CE marking of its TIVUS (therapeutic intravascular ultrasound) ablative catheter device.
SyncVision is currently installed in multiple limited market release sites throughout USA and Europe. According to Volcano, the new system allows co-registration of angiography with intravascular ultrasound imaging.
Ochsner Medical Center has installed a new hybrid operating suite and cardiovascular X-ray system to conduct transcatheter aortic valve replacement and other coronary and peripheral procedures.
Cardinal Health has announced its entrance to the interventional cardiology arena with the purchase of AccessClosure for US$320 million.
A study has found that bivalirudin use during percutaneous coronary intervention (PCI) is associated with less composite bleeding when compared with unfractionated heparin monotherapy, in patients with non-ST segment-elevation acute coronary syndromes or stable ischaemic heart disease.
A study among patients with diabetes, drawn from seven randomised trials, has shown that bleeding within 30 days of percutaneous coronary intervention is associated with an increased risk of one-year mortality, non-fatal myocardial infarction or stent thrombosis.
The Federal Circuit Court of Appeals has granted a request from Medtronic to postpone the implementation of an injunction that would have prevented the company from selling its CoreValve system in the United States.
STENTYS announced that Dianne Blanco has been appointed as a director to replace the representative from Omnes Capital venture capital fund.
Abbott has announced that it has completed enrolment of three clinical trials to support approvals of its Absorb bioresorbable vascular scaffold in the United States, Japan and China. The product, which dissolves over time, will be compared with drug-eluting stents.
Edwards Lifesciences announced it has received CE mark approval for the advanced Edwards Intuity Elite valve system. This next-generation rapid deployment system facilitates smaller incisions in surgical aortic valve implantation (AVI) procedures, and is built upon extensive evidence supporting the durability of the Carpentier-Edwards Perimount heart valve design.
JenaValve Technology announced that it has secured an expansion to its Series C venture round of US$10 million, increasing the total amount raised in the round from US$62.5 million to US$72.5 million.
The Boston Scientific Lotus valve system advanced transcatheter aortic valve implantation (TAVI) technology continued to demonstrate impressive performance at three months, according to new data presented at the American College of Cardiology conference 2014 in Washington, DC, USA.
CardioKinetix announced results of a pooled analysis study of the first-of-its-kind catheter-based Parachute ventricular partitioning device. Twelve-month clinical results from 111 consecutive US and European patients with ischaemic heart failure were presented at the 2014 American College of Cardiology (ACC) Conference in Washington, DC, USA, by Philip Adamson, medical director at the Heart Failure Institute at Oklahoma Heart Hospital.
Data from the PLATINUM Workhorse clinical trial demonstrate low event rates out to four years with Platinum chromium everolimus-eluting stent system confirming excellent long-term performance. At four years, the Platinum chromium everolimus-eluting stent system also continued to demonstrate advantages over the Cobalt chromium everolimus-eluting stent system.
Neovasc reported that final data from its COSIRA trial assessing the efficacy and safety of the Neovasc Reducer, a novel percutaneous device for the treatment of refractory angina, were presented on March 29, 2014 in a Featured Clinical Research Presentation at ACC.14, the American College of Cardiology 63rd Annual Scientific Session & Expo.
The American College of Cardiology (ACC) has elected Patrick O'Gara, as president for the year ahead at its 63rd Annual Scientific Session.
Sorin Group announced Perceval, its sutureless aortic valve has received CE mark approval for adult age indication allowing treatment of a wider spectrum of patients with aortic stenosis and/or steno-insufficiency. Until now, only patients over 65 years of age could benefit from the Perceval technology.
Tryton Medical announced that it has received CE mark approval for the treatment of left main coronary artery disease. With this approval, Tryton Medical becomes the first company to earn a CE mark for this indication.
ACIST Medical Systems announced the global introduction of the new ACIST RXi rapid exchange fractional flow reserve system - the world’s first rapid exchange fractional flow reserve system. This device features new technology designed to provide physicians with a fast and easy way to perform fractional flow reserve procedures.
Medtronic announced the final follow-up results from the CoreValve CE pivotal study, which demonstrated excellent long-term durability at four years in patients with severe aortic stenosis who were treated with the self-expanding CoreValve system.
AccessClosure commercially launched its Mynx Ace vascular closure device, a safe and secure vascular closure product that provides consistent results with a new, easy-to-use deployment system to seal femoral artery access sites.
OrbusNeich has announced the launch of the Sapphire II NC coronary dilatation catheter, a non-compliant balloon catheter engineered to cross tight lesions.
Medtronic announced the one-year results of the Melody transcatheter pulmonary valve US post-approval study, which found that real-world use of the Melody transcatheter pulmonary valve was associated with high procedural success, excellent valve function and few repeat procedures at the primary endpoint of six months.
New pooled analysis of 5,800 patients shows bivalirudin is associated with reductions in cardiac death, major bleeding, transfusion and net adverse clinical events in STEMI patients undergoing percutaneous coronary intervention.
OrbusNeich announced the completion of enrolment in the prospective, multicentre, all-comers REMEDEE registry to evaluate the Combo stent for the treatment of coronary lesions in the setting of routine clinical care.
Medtronic announced that the SYMPLICITY HTN-3 clinical trial, the first and only blinded, randomised, sham controlled study of renal denervation for treatment-resistant hypertension, met its primary safety endpoint but did not meet its primary or secondary efficacy endpoints.
Medtronic announced that the CoreValve system showed results superior to surgical aortic valve replacement at one year in the High Risk Study of its CoreValve US pivotal trial, which evaluated patients with severe aortic stenosis who are considered high risk for surgery.
Sub-analyses of the EXAMINE trial to be presented at the American College of Cardiology's 63rd Annual Scientific Session investigate the effects of alogliptin on cardiovascular mortality rates and hospitalisation for heart failure in type 2 diabetes patients with recent acute coronary syndrome.
Terumo has obtained an exclusive option to purchase Arterial Remodelling Technologies' bioresorbable scaffold technology for use in the treatment of coronary artery disease.
Cardiovascular Systems will feature one-year data from the ORBIT II study of the company's Diamondback 360 coronary orbital atherectomy system.
The key feature of the Joint British Societies Consensus Recommendations on the Prevention of Cardiovascular Disease is an innovative risk calculator: a web-based interactive tool, health professionals can use with patients to help communicate their longer term cardiovascular disease risk.
A study in the Canadian Medical Association Journal suggests that among younger patients with acute coronary syndrome, women have different access to care than men and feminine personality traits (in both men and women) are a risk factor for poorer access to care.
A recent study presented at the American Heart Association annual meeting (16–20 November, Dallas, USA) and published ahead of print in the Journal of the American Medical Association found that early therapeutic hypothermia (prior to hospital; immediately after return of spontaneous circulation) does not improve survival or neurological function in patients who have survived a cardiac arrest.
According to a new study, effective regurgitant orifice area, mitral valve orifice area, and transmitral pressure gradient are independent predictors of acute procedural failure in patients undergoing percutaneous mitral valve repair (MitraClip, Abbott Vascular).
Volcano Corporation has announced US Food and Drug Administration (FDA) clearance of its proprietary instant wave-free ratio (iFR) Modality and immediate commencement of US limited market release.
On-X Life Technologies announced that it has assembled its European sales force at a training symposium in Barcelona ahead of launching its international marketing campaign for the On-X Plus 1.5 aortic heart valve.
The IDE trial will study CeloNova's Cobra PzF, Polyzene-F stent technology in patients with heart disease. It will enrol patients in multiple research centres across the United States and in Europe.
According to the company report, at six-month follow-up, the mean pressure gradient was reduced from 59 mmHG at the start of the study to just 22 mmHG at six months post-implantation. Patients had zero AV-block or new pacemaker, and zero paravalvular leak.
BioCardia announced receipt of the CE mark for its Morph AccessPro steerable introducer, designed for easier navigation through the vasculature during delivery of biotherapeutics and medical devices.
The Neovasc Reducer for refractory angina was featured in a "live case" broadcast at the 10th Annual Congress of Update in Cardiology and Cardiovascular Surgery held in Antalya, Turkey.
Ehrin Armstrong reviews the revascularisation options and medical therapy for patients with diabetes and coronary artery disease.
Boston Scientific Corporation has received CE mark approval for the Rebel platinum chromium coronary stent system, the company's latest generation bare metal stent for the treatment of coronary artery disease.
The remote robotics programme envisions enabling remote robotic-assisted percutaneous coronary intervention for patients in rural areas. The remote robotics programme is the product of a partnership between Corindus Vascular Robotics and Sandford Health and the Leona M and Harry B Helmsley Charitable Trust.
Surgeons in France have successfully replaced the aortic valve in two patients without opening the chest during surgery. The procedure, using totally endoscopic aortic valve replacement (TEAVR), shows potential for improving quality of life of heart patients by offering significantly reduced chest trauma.
A meta-analysis of transcatheter aortic valve implantation (TAVI) studies suggests that the design of a valve (eg. CoreValve vs. Sapien) does not affect the rate of stroke after TAVI. It also found that the approach (eg. transfemoral vs. transapical) also does not affect the incidence of stroke.
Clinical trials awaiting US Food and Drug Administration (FDA) approval will document the use of a new smaller 50cc version of the SynCardia Total Artificial Heart, making it available to people of smaller size, including most women, men and many adolescents.
The combination of aspirin and clopidogrel (or dual antiplatelet therapy) does not significantly reduce the risk of adverse events in aspirin-resistant patients who have undergone coronary artery bypass grafting (CABG) compared with aspirin monotherapy.
The first three human implants of the Fortis mitral transcatheter heart valve were performed in February and March by the Heart Team at St. Thomas' Hospital in London, UK.
The Transcatheter Technologies Trinity TAVI system is designed to be the world's first 'truly repositionable' TAVI system.
Medtronic announced that the European Patent Office has invalidated, in its entirety, the Edwards Lifesciences EP2055266 Spenser patent which was the basis for the August 26, 2013 injunction prohibiting sales of the CoreValve System in Germany.
To improve clinical staff safety during interventional procedures and help monitor radiation dose, Toshiba Medical Systems Europe has partnered with Unfors RaySafe AB to offer a new dose monitoring and management tool for Infinix-i cardiovascular X-ray systems.
Thoratec Corporation initiated a voluntary worldwide Medical Device Correction in order to update its labelling and training materials for the HeartMate II LVAS Pocket System Controller. This safety advisory is being issued because some patients and caregivers have experienced difficulties with the process of changing from a primary system controller to their backup system controller.
Royal Philips will demonstrate advanced image quality, visualisation and clinical decision support at the European Congress of Radiology, March 6-10, in Vienna, Austria. Building on more than 20 years of digital imaging experience, the company is expanding its portfolio of integrated imaging solutions to improve access, optimise workflows and increase quality across the continuum of care.
On-X Life Technologies announced that it will launch its European marketing campaign for the On-X Plus 1.5 aortic heart valve in concert with its Great Britain distributor Vascutek at the Annual Meeting & Cardiothoracic Forum of the Society for Cardiothoracic Surgery in Great Britain & Ireland, March 10-12, Edinburgh, Scotland.
Data presented showed rapid polymer absorption within three months coupled with drug delivery profile up to nine months, providing for excellent healing.
The study showed that use of isosmolar contrast agent Visipaque 320 (iodixanol 320mg I/ml) provides better image quality of coronary stents during multi-detector CT coronary angiography than use of Iomeron 400 (iomeprol 400mg l/ml) when injected at the same flow rate (5.0 ml/s) and volume (80ml).
New practice guidelines for the management of patients with valvular heart disease provide updated definitions of disease severity – categorising four progressive stages from "at risk" to "symptomatic severe" – and lower the threshold for intervention in select patient populations.
Xeltis has announced that it has finished enrolment in a five-patient feasibility study of implantable products intended to enable for the first time the spontaneous growth of natural, healthy heart valves and vessels.
New imaging techniques that can pinpoint the severity of a patient's heart disease are being researched by a team at King's College London thanks to a Novel and Emerging Technologies grant from national heart charity Heart Research UK.
OrbusNeich has initiated the HARMONEE study in Japan to evaluate the Combo dual therapy stent in patients presenting with ischaemic coronary disease and non-ST segment myocardial infarction.
In patients undergoing transfemoral transcatheter aortic valve implantation (TAVI), heparin administration guided by baseline activated clotting time values is associated with significantly less bleeding than heparin administration guided by the patient's body weight alone.
The traditional practice of keeping patients nil-by-mouth for four-to-six hours prior to an elective percutaneous coronary intervention (PCI) procedure may not always be necessary as a retrospective study has found that not fasting before PCI is safe.
Guy MacGowan reviews the benefits of using left ventricular assist devices to improve survival in patients with heart failure, and examines why there seems to be a reluctance to use the devices in the UK.
In a UK first, experts from Glenfield Hospital have repaired a dysfunctional heart valve by inserting a tiny implant measuring just 23mm in size.
The patients admitted to the London Chest Hospital will be among the first of 3,000 individuals involved in the Europe-wide trial to test whether administering a patient's own stem cells shortly after a heart attack will prolong life.
Robbert de Winter, of the Academic Medical Centre, Amsterdam, The Netherlands, presented an update on the REMEDEE Registry, a post-market registry to evaluate the long-term safety and performance of the Combo Dual Therapy stent in routine clinical practice at the Joint Interventional Meeting.
The American College of Cardiology and the American Heart Association have released a mobile and web-based app for health care professionals to use with their patients in determining 10-year and lifetime risks for developing atherosclerotic cardiovascular disease.
One-year data from the ORBIT II study of the Diamondback 360 coronary orbital atherectomy system in treating severely calcified lesions will be presented as an iMPACT Trial at the 2014 Cardiovascular Research Technologies conference in Washington, DC, USA.
Cardiac Dimensions announced that it has received German Neue Untersuchungs und Behandlungsmethoden (NUB) Status 1 approval across 120 leading hospitals in Germany, which may now request reimbursement from their associated insurance companies to cover the costs of Carillon mitral contour system procedures.
The SENTINEL trial to evaluate the safety and efficacy of the Sentinel cerebral protection system for embolic protection during Transcatheter Aortic Valve Implantation has received Investigational Device Exemption (IDE) approval from the Food and Drug Administration (FDA).
Corindus Vascular Robotics announced that cases submitted by users of its CorPath Vascular Robotics system have been accepted for presentation at the Cardiovascular Research Technologies (CRT) conference on February 22 – 25, 2014 in Washington, DC, USA.
Terumo Corporation announced that it has received CE mark approval for its drug-eluting stent, "Ultimaster", with features expected to reduce stent thrombosis and to produce favourable long term clinical outcomes.
Royal Philips has reached its enrolment goal in the SAVE study, which examines the impact of leading obstructive sleep apnea treatment on cardiovascular disease.
Ronald P Caputo writes about robotic systems for percutaneous coronary intervention designed to provide greater accuracy of lesion measurement, aid in better advancement of guidewires and help ensure correct placement of stents.
Enrolment of the 5,000-patient discovery cohort of the GLOBAL (genetic loci and burden of atherosclerotic lesions) clinical study, designed to identify disease-related pathways, new drug targets and biomarkers for cardiovascular diseases, has been completed. Pilot data for the first cohort of patients are expected later this year.
A recent study has found that obesity during pregnancy is an independent risk factor for long-term cardiovascular morbidity. Researchers concluded that obese pregnant patients might benefit from cardiovascular risk screening that could lead to early detection and secondary prevention of cardiovascular morbidity.
More people who have known coronary heart disease die from other causes - such as cancer, and lung and neurological diseases - than heart disease, compared with 20 years ago, according to a Mayo Clinic study published online in Circulation, an American Heart Association journal.
CathMaps+ is the world’s first mobile application for people with an elevated risk of a cardiac incident, which integrates their cardiac history with an interactive map of catheterisation Labs throughout most of the world, and has now been launched in the USA.
A new study indicates using a uniform 14ng/L cutoff for the high sensitivity cardiac troponin T assay, rather than adapting the cutoff for the patient's age or gender, may lead to over diagnosis of myocardial infarction particularly in elderly men.
In a clinical review evaluating cangrelor for reducing thrombotic cardiovascular events during percutaneous coronary intervention, one reviewer says the drug should not be recommended for this indication because studies have failed show that a cangrelor regimen is superior or non-inferior to a clopidogrel regimen or standard of care.
Paravalvular leak after transcatheter aortic valve implantation (TAVI) continues to be a concern, and the question of whether mild paravalvular leak is important remains unanswered. Susheel Kodali speaks to Cardiovascular News about the identification and management of mild paravalvular leak.
Ashok Seth is the immediate past president of Cardiological Society of India and is the principal investigator of the Absorb First registry. He has been awarded the "Padma Shri" by the president of India, one of the highest civilian honours of the Government of India, and talks to Cardiovascular News about performing his father's angioplasty, his mission to make Delhi state a safer place to live, and the importance of planning ahead to avoid complications.
A report published recently provides evidence that the symptoms of depressive disorder are causally associated with the risk of coronary heart disease, and as such should be considered a potentially modifiable risk factor for the occurrence of coronary heart disease.
A study published recently in the Journal of the American Medical Association, reports that in an analysis of blood pressure patterns over a 25-year span from young adulthood to middle age, individuals who exhibited elevated and increasing blood pressure levels throughout this time period had greater odds of having higher measures of coronary artery calcification.
Admedus has received Food and Drug Administration (FDA) clearance to market CardioCel in the USA. CardioCel will be used in pericardial closure and for the repair of cardiac and vascular defects in both adults and paediatrics.
Claret Medical has announced that it has received CE mark for the Sentinel cerebral protection system for embolic protection during transcatheter aortic valve implantation (TAVI). The product will launch immediately in selected CE mark countries.
Edwards Lifesciences has announced that it has received CE-mark approval for its transcatheter heart valve, Sapien XT, to be used for valve-in-valve procedures in both the aortic valve and the mitral valves.
According to the latest UK national audit, the expansion in the use of PCI procedures has meant that patients with acute coronary syndromes are treated more quickly.
Neovasc has announced that a first-in-human implantation of its Tiara transcatheter mitral valve was successfully performed on January 30th by physicians at St. Paul's Hospital in Vancouver.
Vinayak Bapat writes about the key features of his app that provides advice on performing valve-in-valve procedures and explains why he feels such an app is necessary
A study published ahead of print in Circulation: Cardiovascular Interventions shows that the most common cause of readmission after percutaneous coronary intervention is recurrent chest discomfort. However, few patients with recurrent chest discomfort meet the criteria for myocardial infarction.
A survey has found that barely one in four patients who have undergone percutaneous coronary intervention know that they still have coronary artery disease as many patients believe that the procedure cures their condition.
ReCor Medical has announced that it has received CE-mark approval for the latest generation of its ultrasound-based renal denervation system. It adds that the first patients were treated with the new system in December 2013 at the Universitäts–Herzzentrum, Bad Krozingen, Germany.
Transcatheter Technologies has announced it has completed durability testing for its protective aortic heart valve, which is an intrinsic part of the company's transcatheter aortic valve implantation (TAVI) device, Trinity.
Royal Philips has announced the formation of Healthcare Informatics Solutions and Services, a new business group within Philips' Healthcare sector that offers hospitals and health systems the customised clinical programmes, advanced data analytics and interoperable, cloud-based platforms necessary to implement new models of care.
Edwards Lifesciences has announced that it will begin a US trial of its Sapien 3 transcatheter aortic valve implantation (TAVI) device in patients who are at intermediate risk.
Covidien has announced that because of a slower than expected development of the renal denervation market, it is voluntarily exiting its OneShot renal denervation programme.
On-X Life Technologies has announced today that its previously CE-marked On-X prosthetic heart valve has received European regulatory approval for an expanded labelling claim, which now permits the company to market its valve with a reduced requirement for the use of blood-thinning drugs such as warfarin.
Elixir Medical has announced that the first commercial implant of its novolimus-eluting bioresorbable coronary scaffold–DESolve has taken place in Germany. The device is designed to degrade within one year.
Medtronic has announced the FDA approval of its self-expanding transcatheter CoreValve system for severe aortic stenosis patients who are too ill or frail to have their aortic valves replaced through traditional open-heart surgery.
Rebecca Hahn reviews the expanding role of echocardiography in transcatheter aortic valve implantation (TAVI), discussing the use of this imaging modality in treatment planning, intraprocedure guidance, and post-procedure assessment.
A study published ahead of print in Circulation did not find a significant difference in the rate of all-cause mortality at two years between patients who received a permanent pacemaker within 30 days of undergoing TAVI and TAVI patients who did not receive a pacemaker.
BioCardia has announced that it has received the CE mark for its Helix transendocardial delivery catheter, which has been optimised for enlarged ventricles and, therefore, increases the potential patient pool for transendocardial delivery of stem cell therapy.
A health economics analysis published in the Journal of Hypertension suggests that Barostim therapy is a cost-effective treatment option for patients with drug-resistant hypertension
Direct Flow Medical has announced that it has received the CE mark for its fully repositionable 29mm transcatheter aortic heart valve, which is delivered through a flexible, 18-French transfemoral delivery system.
A new guidewire (acWire, MediValve) has received CE mark clearance. The guidewire is intended to facilitate the accurate placement and alignment of medical devices in the cardiovascular system during diagnostic and interventional cardiovascular procedures.
After establishing marketing in Europe and the Middle East, Stentys has announced it is continuing its international development by establishing partnerships with national distributors specialised in cardiovascular products in Singapore, Hong Kong and Malaysia.
Boston Scientific has appointed Craig Thompson as its new senior vice president chief medical officer of interventional cardiology. The company reports that Thompson will play a key role in driving the development of innovative medical solutions within the Boston Scientific interventional cardiology business.
The Medicines Company has announced that the European Medicines Agency (EMA) has accepted for review a marketing authorisation application for the investigational intravenous antiplatelet agent cangrelor.
According to the company, the new institute is a comprehensive resource that provides education and training for physicians treating bifurcated coronary artery disease
The American College of Cardiology, along with nine key specialty and subspecialty societies, has published a document assessing 80 potential clinical scenarios with the goal of assisting physician and patient decision-making.
St Jude Medical has received the CE mark for its 25mm transcatheter aortic valve implantation Portico device, meaning that more patients can now undergo TAVI with the Portico system.
Study author Axel Bauer describes the main findings of a study that investigates whether impaired cardiac baroreflex sensitivity could be used to identify patients who will respond to renal denervation. The study was published ahead of print in the Journal of the American College of Cardiology.
Toshiba Medical has announced that it has become the first medical equipment provider to offer a mobile CT scanner service in the UK. The state-of-the-art Aquilion CXL scanner, a high-end, low dose and fast reconstruction CT scanner was launched at the start of December.
Boston Scientific and The Medicines Company have announced a co-promotion agreement for the Promus Premier stent system. Therefore, The Medicines Company's acute cardiovascular care sales force will collaborate with the Boston Scientific Interventional Cardiology sales force to provide promotional support for the stent system in USA hospitals beginning January 2014.
According to GlobalData, the global renal denervation market will significantly increase from $15.5m (its 2012 level) to $171.7m by 2019. The research and consulting firm say that the expected increase will be a result of the intervention's approval in major countries.
InspireMD has announced that it has appointed Rick Olson, who was previously the director of international strategy and high performance management system at Covidien, as its new vice president of global sales operations.
A study comparing transcatheter aortic valve implantation with minimally invasive aortic valve replacement with a sutureless valve has found that the latter approach is associated with less paravalvular leak, reduced mortality and could possibly be a first-line treatment for (grey-area) patients who fall between surgery and TAVI.
A meta-analysis indicates that percutaneous coronary intervention does not reduce the risk of death, non-fatal myocardial infarction, unplanned revascularisation, or angina compared with medical management alone in patients with stable coronary artery disease and myocardial ischaemia.
Medtronic has announced that it has received both CE-mark approval and Australian therapeutic goods administration (TGA) listing for a new Symplicity renal denervation system, consisting of a flexible 4 French multielectrode catheter (Symplicity Spyral) and a radio frequency generator (Symplicity G3).
Esaote has announced it will demonstrate its prevention suite, a combination of four imaging technologies that enables practical pre-clinical assessment of cardiovascular disease, at the EuroEcho-Imaging conference in Istanbul, Turkey (11-14 December)
A pilot study of a "truly prepositional" transcatheter aortic valve implantation (TAVI) system indicates that the system (Trinity, Transcatheter Technologies) is associated with reduction in mean pressure gradient from 59mmHg at baseline to 20mmHG at 30 days post-implantation.
At the Radiological Society of North America annual meeting (1–6 December, Chicago, USA), Siemens will launch its new Artis one angiography system for universal use. The new system has been optimised for broad clinical use.
HeartWare has announced that it has acquired CircuLite, which developed the Synergy circulation support system for the management of less sick, ambulatory, chronic heart failure patients who are not yet inotrope-dependent.
According to a company release, the Promus Premier stent features customised platinum chromium alloy stent architecture, an everolimus drug with a biocompatible, fluorinated co-polymer, and an enhanced stent-delivery system.
The American College of Cardiology, the American Association for Thoracic Surgery (AATS) the Society for Cardiovascular Angiography and Interventions (SCAI), and The Society of Thoracic Surgeons (STS) have published a joint document on using transcatheter therapies to manage mitral regurgitation
Sorin has announced that it has received the CE mark for the XL version of its Perceval bioprosthetic aortic valve, which is designed to replace a diseased native or malfunctioning prosthetic aortic valve using either traditional or minimally invasive heart surgery.
In the second part of the webcast, Latib presents data demonstrating that DAPT longer than 12 months increases the risk of bleeding; he also talks about differences between first and second generation DES and their impact on DAPT duration. Silber speaks about the results of the RESOLUTE clinical programme.
Alexandre Abizaid, Azeem Latib and Sigmund Silber discuss whether the current US and European dual antiplatelet therapy guidelines for patients treated with drug-eluting stents should be revisited. Flavio Ribichini moderates the discussion. In the first part of this webcast, Abizaid presents the past and current guidelines.
After receiving CE mark approval, Sorin's Solo Smart biological aortic pericardial heart valve has been implanted for the first time. The bioprosthesis is designed to respect the aortic root physiology and ensure a physiological blood flow through the valve annulus.
Adrian Banning charts the evolution of the stent from the original bare metal design to bioabsorbable scaffold and biodegradable polymers. He also looks at the challenges of further improving stent design
Results from a registry suggest that FFR-guided reclassification of the management strategy in patients with ambiguous coronary lesions is safe as it does not adversely affect clinical outcomes.
The results of the XIMA (Xience or Vision stents for the management of angina in the elderly) study indicates that in patients aged ≥80 years, the use of drug-eluting stents is associated less myocardial infarction and target vessel revascularisation than bare metal stents.
A study has indicated that a campless technique, consisting of off-pump coronary artery bypass in combination with the Heartstring proximal seal system, reduces the risk of perioperative strokes during coronary artery bypass grafting (CABG).
After receiving CE mark approval, the Lotus transcatheter aortic valve implantation (TAVI) device has been implanted in two German patients.
Svelte Medical Systems has announced that enrolment in its DIRECT II study, which is assessing the use of its rapamycin-eluting stent with a biodegradable polymer in patients with coronary artery disease, has been completed.
Alvimedica has announced a merger with CID (Carbostent & Implantable Devices). Both companies will now work under the brand name Alvimedica.
A German court ordered the discontinuation, in its entirety, of a prior court ruling that prohibited Medtronic from commercially marketing or selling the CoreValve system in Germany since 26 August 2013.
Sorin has announced that the first European implant of its Mitroflow Valsalva conduit device was performed by Roberto di Bartolomeo, chief of the Cardiac Surgery Department at Sant'Orsola-Malpighi Hospital, Bologna, Italy.
Topline results from a multicentre, prospective, randomised, sham-controlled study indicate that a percutaneous device (Neovasc Reducer) significantly improves functional capabilities in patients with refractory angina.
At a roundtable organised by Cardiovascular News, with an unrestricted education...
Marie-Claude Morice discusses data for CABG vs. data for PCI and how newer generation drug-eluting stents may affect the future treatment of patients with coronary artery disease.
The SAFE-PCI for Women trial suggests that the transradial approach in women undergoing cardiac catheterisation is associated with significantly less bleeding or vascular complications compared with the transfemoral approach.
The one-year results from the SMART-CASE study indicate that a conservative strategy of only performing percutaneous coronary intervention if the diameter stenosis of the target lesion is >70% is non-inferior to an aggressive strategy of performing PCI if the diameter stenosis is >50%.
Cardiovascular News interviews Francesco Bedogni from Sant’Ambrogio Hospital, Mi...
An Investigational Device Exemption pilot study of Therox's next-generation system for supersaturated oxygen therapy indicated that patients who received the therapy had a 9.6% median infarct size at 30 days.
Data presented at TCT suggest that the Self-Apposing stent (Stentys) can be used for indications other than acute myocardial infarction (its current indication).
Biotronik's PK Papyrus covered coronary stent has received the CE mark and can now be used for the management of acute coronary artery perforation.
The Cardiovascular Research Foundation (CRF), New York, USA, and the Uppsala Clinical Research Center (UCR), Uppsala, Sweden, have launched a study (PROSPECT II) and a substudy (PROSPECT ABSORB) to assess the ability of intracoronary near infrared spectroscopy (NIRS) to identify non-flow obstructing vulnerable plaques.
Biosensors has a launched a new size of its Axxess bifurcation drug-eluting stent. The new 4.0x9mm size received the CE mark in September and has been specifically designed for the safe and effective treatment of bifurcation lesions involving large main branch vessels and wide bifurcation angulation.
The FDA has given Medtronic investigational device exemption (IDE) approval to assess the use of renal denervation in patients with moderate uncontrolled hypertension.
Thomas F Lüscher is professor and chairman of Cardiology at the University Heart Center, University Hospital Zurich (Switzerland) and the director of Cardiovascular Research, Institute of Physiology, University of Zurich, Switzerland. He has been involved in many pivotal studies. He spoke to Cardiovascular News about this research and other highlights of his career.
New data announced at Transcatheter Cardiovascular Therapeutics (TCT) Conference demonstrate successful deployment in 120 patients with no severe paravalvular regurgitation at 30 days.
Presented at TCT and published in the Lancet, results of DUTCH PEERS negate the notion of class effect with structural stability of "more flexible and highly deliverable devices".
Symetis has announced that the 1000th implantation of its ACCURATE TA transcatheter aortic valve implantation device has taken place.
Data presented at TCT indicate that compared with a control group, InspireMD's MGuard stent is associated with lower mortality in STEMI patients.
Volcano Corporation announced final results of ADVISE (Adenosine vasodilator independent stenosis evaluation) II, a prospective, double-blind, global, multicentre registry designed to investigate the diagnostic utility of the instant wave-Free Ratio (iFR) Modality in assessing the severity of coronary stenosis.
Three-year data from SYMPLICITY HTN-2, the first and longest-running randomised, controlled clinical trial of renal denervation, continues to demonstrate results consistent with data reported previously at six, 12 and 24-months of follow-up, according to a Medtronic release.
The global sales of drug-eluting balloons across the 10 major markets are expected to witness a significant increase over the coming years, from US$164m in 2012 to US$477m in 2019, at a Compound Annual Growth Rate of 16%.
The results shows treatment with MitraClip provides clinical benefits and improves quality of life for patients with debilitating mitral valve disease who are at prohibitive surgical risk, it states in a press release.
Results from the DISCOVER trial, which were presented at the TCT conference, show that no patients who received the Direct Flow Medical's transcatheter aortic valve implantation (TAVI) system had moderate or severe post-procedural aortic regurgitation at the six-month point.
New clinical data presented at 25th annual Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium show durable outcomes as well as excellent early healing and optimal neointimal suppression out to 24 months following placement of the Combo dual therapy stent (OrbusNeich).
Biotronik Japan has announced enrolment of the first patient in the BIOFLOW-IV study, which aims to verify the efficacy and safety of the Orsiro Hybrid drug-eluting stent. The high quality and efficacy of Orsiro has already been confirmed by three important trials, BIOFLOW-I, -II, and –III, which demonstrated the safety and efficacy of Orsiro.
Juventas Therapeutics announced today that the company has completed enrolment of the STOP-HF trial. STOP-HF is a double-blinded, placebo-controlled, multi-centre trial of its non-viral DNA plasmid therapy JVS-100 for patients with symptomatic heart failure.
Royal Philips and RealView Imaging have announced that they have completed a clinical study that has demonstrated the feasibility of using an innovative live 3D holographic visualisation and interaction technology to guide minimally-invasive structural heart disease procedures.
St Jude Medical has announced new results from the EnligHTN I study, confirming safe, rapid and sustained reduction in blood pressure measurements a year and a half post-procedure.
St Jude Medical announced positive results for the 23–25mm Portico Transcatheter Aortic Heart Valves in the Portico Transfemoral CE mark trial (Portico TF CE Trial). The data was presented at TCT in San Francisco.
Boston Scientific has received CE mark for the Lotus Valve System, the company's advanced transcatheter aortic valve implantation (TAVI) technology. This key approval offers a treatment alternative for patients with severe aortic stenosis who are high risk for surgical valve replacement.
The FDA has approved Abbott Vascular's MitraClip device as a treatment option for patients with significant symptomatic degenerative mitral regurgitation who are at prohibitive risk for mitral valve surgery.
Cardiovascular Systems announced that it has received approval from the US Food and Drug Administration (FDA) to market its Diamondback 360 Coronary Orbital Atherectomy System (OAS) as a treatment for severely calcified coronary arteries.
Data for the self-apposing stent (Stentys) from the APPOSITION IV trial will be disclosed during the upcoming Transcatheter Cardiovascular Therapeutics conference (27 October–1 November, San Francisco, USA).
Medtronic's new aspiration catheter, the Export Advance, has a pre-loaded stylet that has been designed to increase the deliverability and kink resistance of the new device while traversing the vasculature to reach the aspiration site.
A study published in the World Journal of Cardiovascular Diseases indicates that the Scoreflex coronary dilatation catheter (OrbusNeich) is associated with less in-stent late loss compared with a non-compliant balloon.
Results from the Extreme Risk study of the CoreValve US Pivotal Trial will be presented at the annual Transcatheter Cardiovascular Therapeutics (TCT) conference (28 October–1 November, San Francisco, USA), Medtronic has announced.
M Zeeshan Khawaja (The Rayne Institute, King's College London & St Thomas' Hospital, London, UK) reported at PCR London Valves that more than half of patients with significant mitral regurgitation saw an improvement in mitral regurgitation grade after transcatheter aortic valve implantation (TAVI).
A Danish study suggests that although the majority of out-of-hospital cardiac arrests occur at night time, at weekends, or in the evening, accessibility to automatic external defibrillators (AEDs) during these times is limited.
The presentation will include results from the company's multicentre Investigational Device Exemption (IDE) pilot study of a second-generation system that delivers supersaturated oxygen (SSO2) therapy for reduction of infarct size after an acute myocardial infarction.
Biotronik has announced that the first patient, as part of a clinical study, has been treated with its bioresorbable magnesium Dreams scaffold.
The Cardiovascular Research Foundation has announced that John (Jack) Lewin has been appointed as its new president and chief executive officer.
Micell Technologies announced on 8 October that a peer-reviewed article discussing imaging and clinical results of the DESSOLVE I trial of its MiStent Sirolimus Eluting Absorbable Polymer Coronary Stent System (MiStent SES) was accepted for publication on the JACC: Cardiovascular Interventions website.
Bluhm Cardiovascular Institute has announced it has enrolled its first patient in the SALUS trial at Northwestern Memorial Hospital, Chicago, USA, for a movable, non-metallic heart valve.
Royal Philips and Accenture has announced the creation of a proof-of-concept demonstration that uses a Google Glass head-mounted display for researching ways to improve the effectiveness and efficiency of performing surgical procedures.
The study, a part of the EU-funded MitoCare Project, will evaluate the reduction of reperfusion injury by TRO40303 in acute ST-elevation myocardial infarction (STEMI) patients.
After receiving the CE mark for its Xlimus sirolimus-eluting stent, Cardionovum has initiated the international market launch of the coronary stent.
Thirty per cent of percutaneous coronary intervention (PCI) procedures aimed at the recanalisation of chronic total occlusion fail, with the major cause being the inability of the guidewire to cross the target lesion. The Enabler-C Catheter system is designed to offer a new solution for the treatment of these challenging lesions, according to Endocross.
BioVentrix has announced the first-in-man use of its Revivent-TC Myocardial Anchoring System, via Less Invasive Ventricular Enhancement (the LIVE procedure). The procedure was performed by Gintaras Kalinauskas and Gierdrius Davidavicius (both of Vilnius University Hospital in Vilnius, Lithuania).
Chaim Lotan is the director of the Heart Institute of The Hadassah-Hebrew University Medical Center (Jerusalem, Israel) and is also course co-director for the Innovations in Cardiovascular Intervention (ICI) meeting (1–3 December, Tel Aviv, Israel). He spoke to Cardiovascular News about his career highlights and the goals of this year's ICI meeting
Data from a registry study suggest that hypertension provides a protective effect against cerebrovascular events after transcatheter aortic valve implantation.
A study has indicated that percutaneous edge-to-edge mitral valve repair with the MitraClip (Abbott Vascular) can be performed safely and effectively in both patients with central degenerative mitral regurgitation and those with non-central degenerative mitral regurgitation
The first patient has been treated in a new study that evaluating the effectiveness of RenalGuard therapy at preventing acute kidney injury in patients undergoing transcatheter aortic valve implantation (TAVI).
Cardiological Solutions has received two new patents for its range of aortic embolic protection devices, which are designed to prevent stroke and other ischaemic complications.
BioVentrix has appointed Serjan D Nikolic as its new vice president of therapy development. Nikolic will focus on the clinical implementation of the company's existing surgical and future transcatheter product lines for less-invasive ventricular enhancement known as the "LIVE" procedure.
Data presented at PCR London Valves indicate that the Lotus transcatheter aortic valve implantation (TAVI) device is not associated with any cases of moderate or severe paravalvular regurgitation in any patient at six months
JenaValve Technology has announced it has received CE mark approval from European regulators for its transapical TAVI system for the treatment of aortic insufficiency.
Boston Scientific has completed enrolment in the EVOLVE II randomised, controlled clinical trial. The EVOLVE II trial is designed to further assess the safety and effectiveness of the Synergy coronary stent system.
El Camino Hospital (Mountain View, USA) announces that it has enrolled its second patient in a global clinical trial comparing the CoreValve System (Medtronic) with surgical aortic valve implantation in patients with severe aortic stenosis who are at intermediate risk for open-heart surgery.
CardioLogical Solutions reports successful completed in vivo validation of its transcatheter aortic valve implantation (TAVI) access-and-closure device (VasoStitch) and reports its aims to produce a first-in-man clinical study.
Corindus Vascular Robotics has announced that it has launched an extensive study of robotic angioplasty to assess the effectiveness and safety of the robotic-assisted CorPath system
TAVI is mostly performed via a transfemoral or a transapical approach. A relevant number of patients with severe aortic stenosis also suffer from symptomatic carotid artery disease. Rainer Moosdorf describes a new combined surgical approach.
Two studies RESPECT (Randomized evaluation of recurrent stroke comparing PFO closure to establish current standard of care treatment) and the PC Trial failed to show that percutaneous closure of patent foramen ovale (PFO) significantly reduces the risk of recurrent stroke. Bernhard Meier reviews the future role of PFO closure in the light of these studies.
The negative results of the ACCOAST study may mean that the strategy of routinely pre-treating patients with non-ST-segment elevation acute coronary syndromes with a P2Y12 antagonist prior to an invasive procedure needs to be revised.
The RE-ALIGN study shows that dabigatran (Pradaxa, Boehringer Ingelheim) is associated with increased thromboembolic events and bleeding complications, compared with warfarin, in patients with mechanical heart valves.
Keystone Heart's Triguard, a cerebral protection device for use in transcatheter aortic valve implantation (TAVI) procedures, has received the CE mark.
The first two implantations of the second-generation transapical JenaValve transcatheter aortic valve implantation system to be performed outside of Europe have been successfully completed in Canada.
The first patient has been enrolled in the US SALUS clinical trial, which is evaluating Direct Flow Medical's transcatheter aortic valve implantation (TAVI) device.
The Protek17, which is an arterial cannula for use with the TandemHeart temporary circulatory support platform, is now available on the market. It has been designed to improve patient safety and ease of use.
A new high-sensitive troponin test could be used to improve the diagnosis and prognosis of patients presenting with symptoms of a myocardial infarction, and it could be particularly beneficial for women.
The FDA has given Philips approval to market its Epiq premium ultrasound system, which uses nSIGHT technology and anatomical intelligence to address complicated imaging needs of an ageing global population.
The CE mark has been awarded to a new version of GE Healthcare's Vivid E9 cardiovascular ultrasound system. The new version features XDclear technology, which is designed to enhance image quality in 2D, 4D, colour and doppler.
Biotronik has launched new 35mm and 40mm versions of its Orsiro hybrid sirolimus-eluting stent, which has a biodegradable polymer matrix.
Data presented at the European Society of Cardiology annual congress (ESC; 31 August–4 September, Amsterdam, The Netherlands) shows that intravenous cangrelor is associated with a significant reduction in angiographic complications compared with oral clopidogrel.
Apica Cardiovascular has received the CE mark for its platform Access, Stabilisation, and Closure (ASC) system, which allows for the delivery of aortic and mitral valves through the chest wall and apex of the beating heart.
Roche has started a new study TROPICAL-ACS that will evaluate the benefit of personalised antiplatelet therapy in patients who have acute coronary syndrome treated with percutaneous coronary intervention.
St Jude Medical has announced that the next generation of its EngliHTN renal denervation system has received the CE mark. The company reports that compared with the original system, the new system reduces total ablation time by 80%.
A new study has found that compared with men, women have an increased risk of incident cancer after cardiac imaging and procedures. However, their absolute risk is low and the benefits of these interventions outweigh the risks.
According to a new study in EuroIntervention, unlike overlapping sirolimus-eluting stents, overlapping everolimus-eluting stents are not associated with poorer outcomes compared with non-overlapping drug-eluting stents.
Free copies of the latest issue of Cardiovascular News will be at available at the European Society of Cardiology's annual meeting (ESC; 31 August–4 September, Amsterdam, The Netherlands) in the Central Village in the congress centre (Amsterdam RAI).
Allied Healthcare Group has announced the CE mark approval and launch of its CardioCel device, which can be used for the repair and reconstruction of heart defects in both children and adults.
Rox Medical has announced that, with the enrolment of the first German patient, it has crossed the halfway point of its CONTROL-HTN trial an international, multicentre, randomised controlled hypertension trial of its Rox Flow procedure.
The FDA has given HeartWare an investigational device exemption (IDE) supplement to allow the company to start enrolment in an additional patient cohort for its ENDURANCE study, which is evaluating the company's ventricular assist system as a destination therapy for heart failure patients.
CardioKinetix has announced its Parachute ventricular partitioning device has been used in Asia for the first time, with four patients being successfully treated with the novel catheter-based device in Kuala Lumpur, Malaysia.
During the Great Debate at this year's EuroPCR (21–24 May, Paris, France), leading experts in interventional cardiology discussed the use of dual antiplatelet therapy (DAPT) after percutaneous coronary intervention (PCI). The key issues raised were the optimum duration of DAPT with second-generation, drug-eluting stents and the use of new, more potent, antiplatelets.
According to the results of a large multicentre registry, patients who undergo TAVI can suffer a rare and life-threatening complication coronary obstruction. The registry investigated the main baseline, procedural characteristics and management of clinical outcomes in patients who experienced coronary obstruction.
Medtronic announced on 12 August the closing of the acquisition of Cardiocom, in an all-cash transaction valued at US$200 million. Medtronic expects the net impact from this transaction to be neutral to fiscal year 2014 earnings.
Patient enrolment has been initiated in a post-market registry for the Combo dual-therapy stent to evaluate its long-term safety and performance in routine clinical practice. The prospective, multicentre, all-comers REMEDEE registry is expected to enrol 1,000 patients.
Researchers at the University of Reading, UK, have been awarded funding of £114,500 by Heart Research UK to investigate new ways of treating heart failure and factors affecting cell division, survival and death.
Gore has announced a favourable ruling involving the Gore Helex Septal Occluder. Judge Joan Ericksen of the US District Court for the District of Minnesota, USA, ruled on 23 July in Gore's favour that the Gore Helex Septal Occluder does not infringe the asserted claims of AGA Medical US patent and granted Gore's request for summary judgement of non-infringement.
Former US president George W Bush's recent heart stent operation will further increase the popularity of what is often an unnecessary and wasteful procedure, says an analyst with research and consulting firm GlobalData.
Symetis has announced the first-time implantation of its ACURATE TA and ACURATE TF transcatheter aortic valve implantation (TAVI) devices in Japan.
Timothy C Ball, an interventional cardiologist at Carilion Roanoke Memorial Hospital, Roanoke, USA, speaks about using a hybrid operating room and explains a transcatheter aortic valve implantation (TAVI) procedure.
CID has announced that it has received the CE mark for longer lengths 31mm in small sizes and 38mm for the entire product range of its polymer-free, drug-eluting stent, Cre8.
The first prospective pilot study of renal denervation in patients with moderate treatment-resistant hypertension indicates that the intervention is safe and effective in this patient population.
A new study indicates that while both access site bleeding and non-access site bleeding after PCI are independently associated with an increased risk of one-year mortality, non-access site bleeding is a stronger correlate of mortality than access site bleeding
Boston Scientific has announced that it has launched a new imaging coronary imaging catheter, the OptiCross, in Europe and in the USA.
Marc Gillinov and Stephanie Mick, both of the Cleveland Clinic, Cleveland, USA, discuss mitral valve surgery and the options available to patients, including robot-assisted mitral valve repair.
A court in Germany has found that Medtronic's CoreValve infringes Edward Lifesciences' Spenser patent.
Biotronik has launched the Galeo Pro, which is a new range of percutaneous coronary intervention (PCI) guidewires
Corindus Vascular Robotics has announced that for the first time, its CorPath robotic angioplasty device has been used in a PCI procedure.
Sorin has announced that its Vancouver site will manufacture components of the Lotus TAVI valve.
Thoratec Corporation said, in a press release on 1 July, that it has acquired the DuraHeart II ventricular assist system from Terumo for an upfront cash payment of US$13 million and potential future milestone payments, based on regulatory approvals and product sales, of up to US$43.5 million.
Stentys has announced that it has received approval to expand the CE mark indications of its self-apposing coronary stent.
CryoLife announced on 10 July that it has received US Food and Drug Administration (FDA) premarket approval for its new Sologrip minimally invasive laser fibre-optic delivery system.
On the 12 July, Boston Scientific announced it had received US Food and Drug Administration (FDA) 510(k) clearance and CE mark approval for the Guidezilla guide extension catheter and has launched the device in the USA and Europe.
According to an all-comers registry published in EuroIntervention and presented at EuroPCR (21–24 May, Paris, France), cardiogenic shock is the strongest predictor of stent thrombosis following percutaneous coronary intervention.
A small observational study indicates that a self-expanding transcatheter aortic valve implantation (TAVI) device (CoreValve, Medtronic) could potentially be used to treat severe aortic stenosis in patients who have previously undergone mitral valve surgery.
A new NICE guideline aims to improve the outcomes for STEMI patients. The guideline includes recommendations on coronary angiography, primary PCI and reperfusion therapy.
Abbott has announced that the Ministry of Health, Labor and Welfare (MHLW) in Japan has approved the Xience Xpedition everolimus-eluting coronary stent system for the treatment of coronary artery disease.
JenaValve Technology released the first results of its long-term performance and safety in patients with severe aortic stenosis (JUPITER) registry at EuroPCR (21–24 May, Paris, France), demonstrating high procedural success rates and excellent clinical outcomes.
David Holmes (Mayo Clinic, Cardiovascular Diseases and Internal Medicine, Rochester, USA) spoke to Cardiovascular News about the goals of a heart team approach in interventional cardiology
Middle Peak Medical, which is planning to commercialise a novel technology for treating mitral valve disease, has announced that it has US$8.5M in a Series A financing
A study published in the European Journal of Cardio-Thoracic Surgery indicated that 39.6% of patients who received the Revivent Myocardial Anchoring System had a sustained improvement in heart function at one year.
BIBA Medical has announced that it has launched an international TAVI monitor, which is an extension of the company's current TAVI monitor.
Despite white patients and black patients having similar survival rates following aortic valve replacement for aortic stenosis, black patients undergo the surgery significantly less frequently.
StentBoost, a new imaging technique that enhances stent fluoroscopic visibility, is a simple and quick method that offers several advantages during percutaneous coronary intervention in patients with bifurcation lesions
Sachin Goel and Samir Kapadia review the different interventional methods of managing mitral regurgitation
Data from the SEVITENSION study indicate the fixed-dose treatment Sevikar (olmesartan/amlodipine) is superior to perindopril in combination with amlodipine in reducing central blood pressure.
Results from the On-X Heart Valve study presented at the Society for Heart Valve Disease 7th Biennial Congress (22–25 June, Venice, Italy) show that patients on low anticoagulation therapy do well with the On-X prosthetic heart valve.
A new App, for both Mac and Android devices, that provides training on valve-in-valve procedures using transcatheter valves has been launched.
According to several studies presented at the European Society of Cardiology Heart Failure Congress (25–28 May, Lisborn, Portugal), the use of the novel compound Bendavia is associated with improved cardiac function, preserved viable myocardium and increased survival in systolic and diastolic heart failure models.
Medtronic has released the baseline data for the extreme risk patients enrolled in its CoreValve US pivotal trial.
According to data released by Edwards Lifesciences, the Perimount aortic valve is associated with a long-term durability in patients aged 60 or younger at the time of operation.
The joint 2013 European Society of Hypertension/European Society of Cardiology guidelines for the management of arterial hypertension state that lifestyle changes are the "cornerstone" to preventing hypertension.
The FDA has approved Cordis's Adroit 6F guiding catheter for use in various coronary anatomies.
The FDA has given approval to AngioScore to change the instructions for use for its AngioSculpt scoring balloon catheter.
Danny Dvir provides a short summary of practical recommendations that can improve procedural success during valve-in-valve procedures
A new study indicates the cardiac magnetic resonance imaging (MRI) stress testing could be used to predict the risk of cardiovascular death and myocardial infarction in patients with known or suspected coronary artery disease.
A study has found that the transulnar approach for coronary procedures is associated with significantly higher crossover rates compared with the transradial approach.
According to the company the trial will examine the "dissolving" heart device compared to Abbott's drug-eluting stents in patients with coronary artery disease in Japan.
Cardiac surgeons in Germany, Austria and Poland have successfully completed implants and patient enrolment in the VITAL-2 clinical trial for the Vitality Heart Valve System (ValveXchange), according to a company release.
An Oxford-based (UK) master class is available for trainee surgeons to receive training from experience cardiothroacic surgeons and share their own experiences. The class is funded by the Heart UK.
Results from the BIOSOLVE-I study that were presented at the EuroPCR have demonstrated the excellent safety profile of the DREAMS drug-eluting absorbable metal scaffold, according to a company release.
SunTech Medical, in a press release, announced they will be showcasing their ambulatory blood pressure monitoring system for 24 hour management and analysis of patient blood pressure data.
Toshiba has announced that its Infinix DP-i X-ray system is now compatible with its CAT-880B hybrid table for hybrid and transcatheter aortic valve implantation (TAVI) procedures.
According to two European postmarket studies, Abbott Vascular's MitraClip is associated with positive outcomes in patients with mitral regurgitation.
In a study presented at the European Atherosclerosis Society annual meeting (2-5 June, Lyon, France), the first-in-class lipoprotein lipase activator LP071 was shown to reduce plasma levels of triglycerides by almost 100% and more than doubled plasma levels of high-density lipoprotein cholesterol in an animal model.
Biosensors has entered into a licensing agreement with Eurocor on the company’s drug-eluting balloons. The first part of the agreement states that Bionsensors will market and sell drug-eluting balloons manufactured by Eurocor under its own brand.
During a late-breaking trial session at EuroPCR (21–24 May, Paris, France), principal investigator Francesco Prati presented the results of the Demonstr8 study, which showed the Cre8 drug-eluting stent to be non-inferior to an Abbott Vascular bare metal stent with a high statistically significant difference (RUTTS Score <30%; 99.75% Cre8 vs.99.55% bare metal stent; p<0.0001).
Long-term data from the DIVERGE study, presented at EuroPCR (21–24 May, Paris, France), shows that the Axxess drug-eluting stent is associated with safe and effective treatment of complex coronary lesions.
Maquet has announced it has acquired the company LAAx and its TigerPaw system II occlusion device.
Registry data presented at EuroPCR (21–24 May, Paris, France) shows the Neovasc Reducer, a novel percutaneous device that is CE marked for the treatment of refractory angina, to improve cardiovascular parameters in refractory angina patients.
After receiving the CE mark for its dual therapy Combo stent, OrbusNeich has launched the device onto to the European market.
A late-breaking study, ADVISE (Adenosine vasodilator independent stenosis evaluation) II, presented at EuroPCR (21–24 May, Paris, France) indicates that the combination of instant wave-free ratio (iFR) and fractional flow reserve (FFR) is a useful clinical approach for assessing coronary lesions.
Kona Medical presented three- and six-month data from its WAVE I study of its Surround Sound renal denervation system in a late-breaking trial session at EuroPCR (21-24 May, Paris, France).
Results from a large observational study, presented at EuroPCR (21–24 May, Paris, France) suggest that in the absence of GP IIb/IIIa receptor inhibitors, bivalirudin does not improve outcome compared with heparin in patients with non-ST segment elevation acute coronary syndrome undergoing percutaneous coronary intervention.
A late-breaking trial, presented at EuroPCR (21–24 May, Paris, France), indicates that Biotronik's Orsiro a drug-eluting stent with a biodegradable polymer was non-inferior to Abbott Vascular's Xience Prime, a drug-eluting stent with a durable polymer, in terms of in-stent late lumen loss.
Daniel Steinberg reviews the transradial approach in primary percutaneous coronary intervention (PCI) and looks at why the USA has been reluctant to adopt the approach despite it reducing access site and bleeding complications compared with the transfemoral approach
Renu Virmani, medical director, CVPath Institute, Gaithersburg, USA, spoke to Cardiovascular News about bioresorbable stents and their potential impact on the future management of coronary artery disease.
Morton Kern is chief of cardiology at the Long Beach Veterans Administration Hospital, Long Beach, USA, and is also associate chief of Cardiology at University of California, Irvine, Orange California, USA. He is the co-chair of the Society for Cardiovascular Angiography and Interventions' annual meeting (SCAI; 8–11 May 2013, Orlando, Florida). He talked to Cardiovascular News about his career and his achievements
Interim data from the REDUCE-HTN study, which was presented at EuroPCR (21–24 May, Paris, France), indicates that the Vessix renal denervation system (Boston Scientific) is associated with a significant and sustained reduction in blood pressure.
Presented at EuroPCR, the one-year results from BELLO study showed durability following treatment of de novo lesions with the novel angioplasty device.
CardioKinetix has announced positive clinical data that showed positive results for patients treated with minimally invasive structural heart device for heart failure.
At EuroPCR 2013 in Paris, France (21–24 May), three separate analyses have addressed one of the most common clinical challenges in the treatment of complex coronary artery disease and showed that the the Resolute drug-eluting stent from Medtronic has performed strongly in coronary bifurcation lesions over the long term.
JenaValve Technology released the first results of its long-term performance and safety in
patients with severe aortic stenosis (JUPITER) registry at EuroPCR (21–24 May, Paris, France), demonstrating high procedural success rates and excellent clinical outcomes.
Direct Flow Medical has announced that it met its primary endpoint in the fully-enrolled, 100-patient DISCOVER CE mark trial by achieving 99% freedom from all-cause mortality at 30 days.
According to a press release clinical evidence supporting the benefits of using the MGuard EPS in STEMI patients continues to grow and a presentation at EuroPCR meeting added important new data underlining the protective benefits of MGuard six months post procedure.
According to a presentation at EuroPCR, "real world" ADVANCE study has confirmed that using CoreValve has good economic value in patients unable to undergo surgery.
According to Abbott, Xience Xpedition is the world's longest coronary drug-eluting stent and has received the CE mark.
Of those studied, only 4.3% were reported as experiencing a primary endpoint event in the e-BioMatrix study.
The ORBIT II trial evaluating safety and effectiveness of the orbital atherectomy system in treating calcified coronary lesions is expected to presented as late-breaking data at EuroPCR (21–24 May, Paris, France).
One-year results from SOURCE XT, one of the largest, post-approval transcatheter aortic valve implantation (TAVI) registries (according to a EuroPCR press release) has reported good clinical outcomes in clinical practice.
The DESolve Nx trial treated target lesions in 126 patients with single de novo coronary artery lesions with the DESolve device. Results showed the primary endpoint of in-stent late lumen loss was 0.21mm (+0.34) at six months.
According to a Medtronic press release, data from the global SYMPLICITY registry showed renal denervation with the Symplicity system met performance expectations in a real-world setting.
According to an online survey of 141 interventional cardiologists worldwide, the use of drug-eluting stents and the delay to healing is a concern.
Philips announced that their CX50 xMATRIX now has 2D intracardiac echo capability. The CX50 xMATRIX with available Live 3D TEE and ICE is expected to be shown in Paris, France, at EuroPCR, 21–24 May.
According to a clinical study and Philips, using the AlluraClarity live guidance system, a 50% reduction in X-ray dose could be achieved without any loss in image quality.
Data of the randomised JACK-EPC trial showed that the Genous stent had a significantly lower rate of binary restenosis compared to bare metal stents in acute coronary syndrome patients. The results have been published in Minerva Cardioangiologica.
Medtronic announced that its Export Advance aspiration catheter recently received the CE mark and will soon be launched in Europe and other international markets.
Published in Circulation, new research examining the frequency and composition of embolic debris captured in TAVI, has found that in a 40 patient series, there were visible debris in 75% of patients when using the Claret Montage Dual Filter System.
A symposiumat EuroPCR (21–24 May, Paris, France) is expected to discussed the Cre8 stent. According to a company release, this will include presentations from clinicians on Cre8 trial data, followed by a panel and audience discussion.
On the 20 May 2013, Biosensors International announced it has received CE mark approval for its BioMatrix NeoFlex, the latest addition to the BioMatrix family of drug-eluting stents.
Circulite has announced it has received CE mark approval to conduct a multicentre trial on the Synergy IC circulatory support system. An abstract on the system is expected to be presented at EuroPCR (21–24 May, Paris, France).
A new study suggests that transcatheter aortic valve implantation (TAVI) devices are under used in Europe, and that this under use is associated with economic status and reimbursement strategies.
On-X Life Technologies has announced that it has completed enrolment for low-risk aortic valve and mitral valve patient groups in its Prospective randomised On-X Valve anticoagulation trial (PROACT).
Elixir Medical has announced it has received Food and Drug Administration (FDA) approval to start its EXCELLA clinical trial of its Desyne Nx novolimus-eluting stent system.
Sorin has announced that it has received conditional approval from the Food and Drug Administration (FDA) to conduct an investigational device exemption (IDE) study of its Perceval S Sutureless aortic valve.
A new risk score, developed using data from the RISK-PCI study, could be used to identify which patients are at high risk of bleeding after undergoing a percutaneous coronary intervention (PCI) procedure.
A study published in Circulation indicates that compared with white patients, black and Hispanic patients have worse outcomes following percutaneous coronary intervention (PCI).
According to a study presented at EuroPRevent 2013, mental vulnerability is associated with a significantly increased risk of fatal and non-fatal cardiovascular disease independently of classical risk factors.
The REMEDEE study, which showed that OrbusNeich's Combo dual therapy stent is effective at controlling neo-intimal proliferation, has been published in the journal JACC: Cardiovascular Interventions.
Symetis has announced that a post-market study has confirmed that its transcatheter aortic valve implantation (TAVI) Acurate TA device is associated with a high procedural success rate.
Results from the C-CURE study, which reviews the use of C3BS-CQR-1 in patients with chronic heart failure, have been published in the Journal of the American College of Cardiology.
Maquet has received both FDA approval and CE mark approval for its AIR-BAND radial compression device.
In March, Natec Medical received a licence to market its Tamarin Blue and Filao RX in Canada
MediLive has developed a 3D training series for both students and medical professionals.
Max Baghai and Olaf Wendler review the current data for TAVI procedures and discuss how the complications of these procedures can be minimised.
A study published ahead of print in the Journal of the American College of Cardiology indicates that following implantation of a left ventricular assist device, sexual function does not improve and may even worsen.
A new study, published ahead of print in the Journal of the American College of Cardiology, indicates that renal denervation delivered through extracorpeal high-intensity focused ultrasound may be an alternative approach to managing resistant hypertension.
BioControl Medical has announced that the FDA has approved full expansion of the INNOVATE-HF study, which is investigating BioControl Medical's CardioFit device for vagal nerve stimulation.
Maquet Medical Systems has announced that it has launched the MIRA-iCS Retractor system, for use during minimally invasive revascularisation procedures, on to the USA market.
Arthesys has announced that it has received the CE mark for its fourth generation Maya coronary balloon catheter.
Bayer has announced that the European Medicines Agency has recommended that its drug rivaroxaban (Xarelto) be approved for the prevention of atherothrombotic events in patients with acute coronary syndromes (ACS).
NeuroVive has announced the 600th patient has been recruited to its pivotal Phase III European, multicentre trial (CIRCUS trial), which is assessing CicloMulsion.
Boston Scientific has announced that the Platinum China study, which was presented at China Interventional Therapeutics (20–23 March, Beijing, China), indicated that the Promus Element stent is associated with a low rate of stent thrombosis at nine months.
The UK's National Institute for Health and Clinical Excellence (NICE) has launched a new quality standard for hypertension, which states that person-centred care is vital in the management of the condition.
The FDA Circulatory System Devices Panel of the Medical Devices Advisory Committee voted five to three that the benefits of Abbott Vascular's MitraClip outweigh its risks.
A new study, published ahead-of-print in the Journal of Thoracic and Cardiovascular Surgery, suggests that the Trifecta aortic valve has a similar haemodynamic performance to that of a healthy aortic valve.
For the first time, the Promus Premier everolimus-eluting coronary stent has been implanted into patients in Ireland and in the Netherlands.
At ACC, data from the ORBIT II study showed that patients treated with the Orbital atherectomy system (Cardiovascular Systems) were associated with an 89.8% freedom from major adverse cardiovascular events rate.
According to the company, the Aquilion ONE ViSION can image the heart in one rotation with a low dose of radiation when used in conjuction with the company's Vital Images' CT Myocardial Analysis software. A trial conducted by the National Institutes of Health confirms in 107 patients that the system's average radiation dose was low.
At the American College of Cardiology Scientific Session (ACC.13), Philips introduced its portfolio of cardiology solutions. This included the IntelliSpace ECG, the ST80i Stress Testing System, the EchoNavigator, and the Corindus CorPath 200 System.
Despite a range of safe and effective antihypertensive therapies, hypertension is still a challenging condition to control only a small percentage of hypertensive patients achieve adequate blood pressure control. Intravascular renal denervation may be beneficial in treating resistant hypertension, but there are gaps in the evidence base. Jan Staessen reviews the current research for this intervention and the priorities for future research
The midtem results from cohort A of the PARTNER study indicate that, after three years, the Sapien transcatheter aortic valve is still structurally intact and that transcatheter aortic valve implantation continues to be comparable to surgery in high-risk patients.
New study, CHAMPION PHOENIX, indicates that cangrelor significantly reduces the rate of ischaemic events including stent thrombosis compared with clopidogrel in patients undergoing urgent or elective percutaneous coronary intervention.
Two-year data for the Symplicity renal denervation system showed that the intervention was associated with a sustained significant drop in blood pressure.
One-year data from APPOSITION III, which is assessing the long-term performance of the Stentys Self-Apposing stent, indicated that the device is associated with a very low rate of mortality (2%).
Results presented at the ACC for the bioresorbable vascular scaffold Absorb showed that the device was associated with a major adverse cardiovascular events rate of 10% at three years.
Results of the DISCOVER trial, presented at ACC 2013, have shown that patients treated with the Direct Flow Medical Transcatheter Aortic Valve System (Direct Flow Medical) achieved excellent survivability and sustained haemodynamic improvements with minimal occurrence of aortic regurgitation at six months.
Results from the IMPACT trial, presented at ACC 2013, have found that cardiologists changed their diagnostic testing strategy of coronary artery disease in 60% of female patients following Corus CAD testing.
At the ACC 2013 sessions, Ian Meredith, Melbourne, Australia, reported clinical endpoint data from the PLATINUM workhorse clinical trial comparing the safety and effectiveness of the Promus Element Coronary Stent System (Boston Scientific) to the Xience V Everolimus-Eluting Coronary Stent System (Abbott).
Data from the Global RESOLUTE clinical programme indicates that interrupting dual antiplatelet therapy one month after a patient is implanted with a Resolute drug-eluting stent is not associated with an increased safety risk.
New data for the MitraClip system indicates that the device is associated with a significantly lower rate of 30-day mortality compared with open surgery.
The study is aimed to evaluate an enhanced version of the Carillon Mitral Contour System (Cardiac Dimensions), a minimally-invasive therapy designed to treat heart failure patients suffering from functional mitral regurgitation.
The Ilumien Optis system combines fractional flow reverse and intravascular optical coherence technology (OCT) to help with stent placement and has been launched by St Jude Medical in Japan.
Siemens Healthcare has announced that the FDA has approved its Artis Q and Artis Q.zen angiography system families.
CircuLite has announced it has received conditional FDA approval for an investigational device exemption study of its Synergy circulation support system.
Rox Medical has announced that the first patients in Ireland have been enrolled in the in CONTROL-HTN hypertension at Beaumont and Mater private hospitals.
Royal Philips Electronics has announced that it has received 510(k) clearance from the FDA to market its EchoNavigator live image-guidance tool.
Medtronic has been given the go-ahead to update the dual antiplatelet therapy CE mark labelling for its Resolute Integrity drug-eluting stent.
The FDA has approved two Humanitarian Use Device (HUD) designations for the new SynCardia's 50cc total artificial heart
Medtronic has announced it has received FDA approval to conduct an early feasibility study of its native outflow tract transcatheter pulmonary valve.
Neovasc has announced that the clinical protocol for its COSIRA study, which assesses the company's novel percutaneous device for reducing refractory angina, has been published in a peer-reviewed journal.
Medtronic has received the CE mark for its Engager transcatheter aortic valve implantation system, which delivers the Engager valve transapically.
Stefan D Anker is the 2012–14 president of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). He has been credited with establishing cachexia as an independent prognostic factor for cardiovascular illness, and he has shown that iron deficiency is a valid therapeutic target in heart failure. He spoke to Cardiovascular News about his career, research in heart failure and his goals during his HFA presidency tenure.
Stefano Savonitto, director, Cardiology Division, Arcispedale S Maria Nuova, Reggio Emilia, Italy, talked to Cardiovascular News about treating older patients with STEMI. He spoke about this topic at JIM.
According to a new study, transcatheter aortic valve implantation is feasible in non-surgical patients with severe native aortic valve regurgitation without stenosis.
Cardiovascular Systems presented three-year data for its orbital atherectomy system at CRT 2013 (23–26 February 2013, Washington, USA).
A new study indicates that microvascular resistance, contrary to previous studies, increases after percutaneous coronary intervention and this increase is not linked to increasing epicardial stenosis.
The FDA has approved the 34mm and 38mm lengths of Medtronic's Resolute Integrity drug-eluting coronary stent.
Direct Flow Medical has been awarded two patents, one from the US Patent and Trademark Office and one from the Japanese Patent Office, for its transcatheter aortic valve implantation system.
According to a study published in Circulation: Cardiovascular Genetics, the Corus CAD test outperforms myocardial perfusion imaging in overall diagnostic accuracy for obstructive coronary artery disease.
St Jude Medical has announced it has initiated a study to evaluate whether the renal denervation can lower the risk of major cardiovascular events as well as lower blood pressure
The IDE study is designed to explore alternative access routes with transcatheter aortic valve implantation (TAVI). It is believed to be a first for any medical specialty society.
ReCor Medical has announced that it has begun ACHIEVE a post-marketing study of its next-generation, ultrasound-based renal denervation system, Paradise.
Intracoronary administration of progenitor cells, in patients with acute myocardial infarction or patients with chronic heart failure, has an adequate safety profile
The Resistant Hypertension Course, a joint initiative of the European Society of Hypertension (ESH), the European Association of Percutaneous Cardiovascular Interventions (EAPCI) and PCR, will take place this week.
José M Pérez-Pomares, member of the European Society of Cardiology (ESC) Working Group on Development, Anatomy and Pathology, reviews the effects of ischemic heart disease and discusses the biological foundations of cell-based approaches to repair the damaged (infarcted) heart.
Despite the perception that financial conflicts of interest influence the outcome of trials, a new study indicates that self-reported financial conflicts of interests are not associated with an increased likelihood of favourable results.
Cardiorentis has initiated the first-ever acute heart failure (AHF) Phase III trial to be specifically designed to assess the effect of early treatment on cardiovascular mortality.
Information about OrbusNeich's Combo dual therapy stent will be presented during the Joint Interventional Meeting (JIM; 14–16 February, Rome, Italy)
According to a company release, the Promus Premier (Boston Scientific) is designed to provide physicians improved drug-eluting stent performance in treating patients with coronary artery disease.
The UK's National Institute for Health and Clinical Excellence (NICE) has issued a draft version of its guideline for acute management of ST-segment-elevation myocardial infarction (STEMI) for public consultation
The first patient in Svelte Medical Systems's DIRECT II (Direct Implantation of Rapamycin-Eluting stents with bioabsorbable drug Carrier Technology) study, the company announced, has been treated.
Therox has announced it begun an investigational device exemption study of its next generation super saturated oxygen therapy for patients with acute myocardial infarction.
St Jude Medical has announced that the first patient in its study evaluating the 25mm Portico transcatheter heart valve has been implanted with the device.
Kips Bay Medical has announced that the first patient in its US clinical feasibility trial has been implanted with the eSVS Mesh graft.
According to Natec Medical, the Tamarin Blue PTCA RX dilatation catheter features exceptional trackability and pushability, and a unique crossability into the tight stenosis with its tapered tip.
Philippe Pibarot reviews the use of stress imaging to determine the severity of aortic stenosis and its management. He spoke about this topic at PCR London Valves (30 September–2 October 2012, London, UK).
A new study indicates that post dilation, following balloon expandable TAVI, is a safe and effective way to reduce paravalvular regurgitation
This post-market clinical study will further evaluate the safety and efficacy of the EnligHTN Renal Denervation System (St Jude Medical) in patients with uncontrolled hypertension.
Survival benefit found with surgical ventricular reconstruction plus coronary artery bypass graft procedure
The CE mark approval for BioFreedom, a polymer-free drug-coated stent, was supported by strong data from the BioFreedom first-in-man study.
According to a press release, the Direct Flow Medical Transcatheter Aortic Valve System is designed to virtually eliminate aortic regurgitation by allowing complete assessment of haemodynamic performance, repositioning and retrieval after the valve is fully deployed in the native valve annulus.
A team at Peter Munk Cardiac Centre, University Health Network, Toronto, Canada,...
According to a company release, the treatment parameters of this new device have been further enhanced to reduce energy delivery to 30 seconds by maximising cooling of the endothelium and efficiently treating the nerves circumferentially.
The objective of this study is to demonstrate the initial safety and performance of the eSVS Mesh for use as an external saphenous vein graft support device during coronary artery bypass surgery.
The company announced that this new code will provide additional reimbursement for the enCorSQ, a new approach to the long-term treatment of mitral valve disease.
Conventional thinking has been that CPR is futile after 20 minutes, but researchers in the USA have found that their study results challenge that assumption.
OrbusNeich is holding a symposium about its COMBO stent at AsiaPCR/SingLIVE 2013 (24–26 January, Singapore)
Placed percutaneously, a company press release explains, OneShot delivers radiofrequency energy in a circumferential manner to the renal arterial wall, and requires only a single treatment per artery.
The HeartMate II Risk Score, as outlined in the Journal of the American College of Cardiology, could be used to estimate mortality in patients receiving a HeartMate II left ventricular assist device
According to a study published in Catheterization and Cardiovascular Interventions, ambulatory percutaneous coronary intervention in patients with stable coronary artery disease is safe and effective
CardioLogical Solutions has been given an additional patent for its aortic embolic protection devices
Phoenix Cardiac Devices states a new study will assess the safety and efficacy of its BACE device for the treatment of mitral regurgitation with the goal of obtaining CE Mark approval
The Pharmacovigilance Risk Assessment Committee of the European Medicines Agency has recommended that the marketing, supply and authorisations for the laropiprant/nicotinic acid containing drugs Tredaptive, Pelzont and Trevaclyn be suspended
A 39-year-old female with New York Heart Association (NYHA) class III heart failure has become the first patient to be implanted with the CardioKinetix's Parachute device in the company multicentre PARACHUTE IV trial
Following on from the use of percutaneous technology in peripheral vascular disease and in coronary artery disease, percutaneous technology is now being used to treat valvular dysfunction. Jose L Navia and Sharif Al-Ruzzeh review its application in this context
As well as receiving the CE mark for its DS1000 mitral valve repair device, NeoChord has also started a 50-patient registry to assess the first European procedures with the device
Abbott's next-generation Xience Xpedition drug-eluting stent system has received FDA approval and has been launched onto the US market
ACES is a randomised study designed to demonstrate the positive clinical benefit and impact on resource utilisation of the Svelte Acrobat Integrated Delivery System (IDS) compared with conventional stent devices.
The TRIS (TandemHeart to Reduce Infarct Size) trial will evaluate the effectiveness of ventricular unloading on the reduction of infarct size for patients who have suffered a severe heart attack.
Study aims to improve clinical outcomes by offering physicians more information to further understand how FFR and OCT influence physician decision-making for coronary intervention
Cosmo Godino, Cardio-Thoracic-Vascular Department, San Raffaele Hospital, Milan, Italy, writes that not all efforts should be directed toward technical and procedural aspects to achieve final chronic total occlusion-PCI recanalisation.
The world's first low profile, 14 French pre-mounted, pre-crimped, and pre-packaged, ready-for-use, TAVI system reduces time from sterile package to valve deployment.
Published data demonstrated that the Symplicity renal denervation system provides blood pressure reduction in patients with treatment-resistant hypertension sustained to 12 months.
The BIOHELIX-I trial evaluates the safety and performance of the PRO-Kinetic Energy coronary, bare metal stent.
Biosensors has announced the enrolment of the first patient in the LEADERS FREE study involving BioFreedom, the polymer-free drug-coated stent.
Celladon has announced that it has dosed the first European patient in its ongoing international Phase 2b clinical trial of MYDICAR.
Matthew Gillespie, Philadelphia, USA, and others have found that transcatheter pulmonary valve implantation with the Melody valve (Medtronic) may be an effective treatment option for patients with failed bioprosthetic valves.
A study published ahead of print in the Journal of the American College of Cardiology indicates that the rates of readmission following axial flow left ventricular assist device (LVAD) implantation decrease during the first six months and thereafter stabilise.
Long-term studies (21 years) and 30 years of clinical use of the Mitroflow valve have demonstrated excellent haemodynamic performance, great durability and ease of use.
Revivent (BioVentrix) offers an alternative less-invasive option than surgical ventricular restoration to repair the left ventricle from damage done by a heart attack, enhancing performance of the heart's non-damaged myocardium.
EVOLVE II is designed to further assess the safety and effectiveness of the Synergy Stent System (Boston Scientific) and support FDA and Japanese regulatory approvals for the treatment of atherosclerotic coronary lesions.
The new survey was undertaken by the American Medical Group Foundation, and released at the launch of the US healthcare campaign "Measure Up, Pressure Down".
ORBIT II is evaluating the safety and effectiveness of Cardiovascular Systems' atherectomy technology in treating severely calcified coronary arteries.
The funding will be used to further validate the company's transcatheter mitral valve implantation technology and is expected to carry the company through its feasibility and CE mark clinical trials.
Results from the BELLO study indicate that the use of a paclitaxel-eluting balloon for the treatment of de novo small vessel disease is associated with less angiographic late loss and similar rates of restenosis and revascularisation compared with a paclitaxel-eluting stent.
Thomas Pilgrim, Bern, Switzerland, and others reported in Circulation: Cardiovascular Interventions that intercurrent events, such as access and bleeding complications, increase the cumulative risk of stroke and death after TAVI.
The PROMISE study will test comparative effectiveness of anatomical versus functional non-invasive diagnostic tests for the assessment of stable symptomatic patients with possible coronary artery disease.
The Journal of the American College of Cardiology (JACC): Heart Failure will begin publishing in February 2013 on a bimonthly basis.
For the first time, the National Cardiovascular Data Registry (NCDR) has published in the Journal of the American College of Cardiology a general report of data from the CathPCI registry, which characterises the clinical profile and outcomes of patients undergoing cardiac catheterisation and percutaneous coronary intervention procedures. Lead author of the report, Gregory Dehmer, reviews the report's key findings.
NICE has recommended ivabradine as an option for the treatment of people with chronic heart failure (NYHA class II to IV) with systolic dysfunction, who are in sinus rhythm and whose heart rate is 75 beats per minute or more and who have a left ventricular ejection fraction of 35% or less.
The Tryton pivotal study is an international randomised trial that compares a Tryton stent in the side branch vs. conventional provisional stenting in the side branch, with both arms of the trial utilising a standard drug-eluting stent in the main vessel.
According to a company release, in both the Artis Q and Artis Q.zen series, the new X-ray tube can help to identify small vessels up to 70% better than conventional X-ray tube technology.
The transapical procedure was performed by Anson Cheung, associate professor of surgery and director of cardiac transplant at St Paul's Hospital in Vancouver, Canada.
Sync-Rx develops advanced software applications that optimise and facilitate transcatheter cardiovascular interventions using automated online image processing.
Cardio3 BioSciences has announced it has received authorisation from the Belgian Federal Agency for Medicines and Health Products (FAMHP) to begin its CHART-1 (Congestive heart failure cardiopoietic regenerative therapy) European phase III trial for C3BS-CQR-1, an autologous stem cell therapy for heart failure, in Belgium.
David Holmes, past president of the American College of Cardiology, principal investigator of the PROTECT AF trial and other several other studies in electrophysiology and interventional cardiology, spoke to Cardiovascular News about his career, mentors, research and interests outside medicine.
The approval was supported with data from the ADVANCE study, in which patients who received the HeatWare device achieved a 94% survival at six months. The trial also met its primary endpoint of establishing non-inferiority between the investigational device and comparator arm of the study.
Corus CAD (CardioDX) is a decision-making tool that can help physicians exclude obstructive coronary arterial disease as the cause of a stable non-diabetic patient's symptoms.
The valve that can be completely resheathed, repositioned or retrieved is designed for patients with severe aortic stenosis who are considered to be inoperable or high risk for conventional open-heart valve replacement surgery.
"the Gore REDUCE clinical study shares notable similarities to the RESPECT study which we feel will help lead to a beneficial study outcome," said Gore REDUCE principal investigators.
The SURTAVI trial will evaluate whether the CoreValve system (Medtronic) is non-inferior to surgical aortic valve replacement, based on the composite primary endpoint of all-cause mortality and disabling stroke at 24 months.
The Federal Circuit Court of Appeals has affirmed the April 2010 jury verdict from the Federal District Court of Delaware that the CoreValve System infringed a single Andersen patent held by Edwards Lifesciences.
Gregory W Stone, New York, USA, presented one-year results, at TCT, from a substudy of the largest study of its kind the ADAPT-DES registy showing that stent procedures guided by intravascular ultrasound (IVUS) resulted in better patient outcomes and fewer complications at 30 days and 12 months compared to procedures without IVUS, and were safe.
This catheter is specifically designed to facilitate quick and accurate thrombus aspiration in myocardial infarction patients with ST-segment elevation.
Improvements in haemodynamics and quality of life were demonstrated at 24 months in an extended analysis of the pilot study which assessed vagus nerve stimulation therapy for heart failure patients with the CardioFit System (Biocontrol Medical).
Reporting on the results of an economic analysis of the FAME II study, at TCT, William Fearon, Stanford, USA, said that fractional flow reserve (FFR) guided percutaneous coronary intervention (PCI) was cost-effective, compared with medical management, in patients with stable coronary disease (CAD).
In the 86.3% of non–ST-elevation acute coronary syndrome (NSTE-ACS) patients with elevated hs-TnT, ticagrelor (Brillinta, Aztrazeneca) tablets reduced the composite of cardiovascular death, myocardial infarction, and stroke.
Results showed that ticagrelor plus aspirin, compared to clopidogrel plus aspirin, reduced cardiovascular death, myocardial infraction and stroke in acute coronary syndrome patients regardless of coronary artery disease extent and treatment strategy.
The objective of this study is to demonstrate the initial safety and performance of the eSVS Mesh for use as an external saphenous vein graft (SVG) support device during CABG procedures.
Vessix Vascular is the developer of the Vessix Vascular V2 Renal Denervation System which has received CE mark in Europe and TGA approval in Australia.
Jay H Traverse, Minneapolis Heart Institute at Abbott Northwestern Hospital, Minneapolis, USA, and colleagues have found that timing of cardiovascular cell delivery within the first week after a heart attack does not have any effect improving left ventricular function following reperfusion.
The new heart device is used for simultaneous implantation of an aortic tissue valve and ascending aorta. It allows for safer device manipulation during assembly and implantation, thus limiting cardiac surgery time.
The study met its primary endpoint showing the MGuard Embolic Protection Coronary Stent was significantly superior to the control arm of bare metal and drug-eluting stents in the treatment of heart attack patients.
Joshua M Hare, Miami, USA, and colleagues have found that "transendocardial injection of allogeneic and autologous mesenchymal stem cells were both associated with low rates of treatment-emergent serious adverse events, including immunologic reactions in patients with left ventricular dysfunction due to ischaemic cardiomyopathy.
The move strengthens Medistim's commitment to the UK market following NICE recommendation of its VeriQ flow measurement system for assessing graft blood flow during coronary artery bypass graft surgery.
A comparative study has shown, at five years, that diabetic patients who underwent bilateral internal thoracic artery grafting had a 13% greater survival rate, as well as a lower risk for non-fatal heart attacks, recurrent chest pain, and future re-interventions, compared to patients treated with percutaneous coronary intervention.
Synergy (Boston Scientific) features a PLGA polymer and everolimus drug coating that dissipates by three months.
A large comparative registry undertaken in a "real-world" setting has shown that, at five years, coronary artery bypass grafting demonstrated lower rates of death, myocardial infarction and target vessel revascularisation compared to percutaneous coronary intervention in patients with left main coronary artery or multivessel disease. Results were presented at EACTS.
First 30-day results of the Engager European pivotal trial support the safety and performance of the Engager transapical aortic valve system (Medtronic) in patients with severe aortic valve stenosis at high risk for surgical aortic valve replacement.
Non compliant percutaneous transluminal coronary angioplasty (PTCA) balloons are commonly used to post-dilatate stents and to treat in stent restenosis.
The Biotronik Pantera Lux Paclitaxel Releasing Balloon is a novel treatment for restenotic coronary artery lesions after drug-eluting or bare metal stenting.
This new fibre-optic device will allow clinicians to provide a higher-efficacy, faster intra-aortic balloon counterpulsation therapy to patients 5'0" to 5'4" tall.
Data presented at TCT show that the primary analysis was not statistically significant but trended towards superiority with the Amplatzer device, while additional analyses demonstrated superiority.
Target lesion revascularisation rates at three years were significantly lower in favour of the DESyne stent as compared to the Endeavor zotarolimus-eluting stent (1.4% vs. 9.9%; p=0.008).
The VidaEcho system features a simulated 3D anatomy of the heart, and the corresponding echo image; by moving a virtual echo probe handle with a mouse, users learn how the device will interact with the cardiac anatomy.
MGuard Embolic Protection Stent (InspireMD) shows a significant 29% improvement in complete ST resolution compared to bare metal or drug eluting stents.
Contrary to previous data, STEMI patients with prior open heart surgery, or coronary artery bypass graft surgery (CABG) and receive a coronary stent have similar outcomes to patients without previous CABG, based on study of a large, prospective, regional STEMI network, presented at TCT.
The Revivent system is intended for patients suffering from ischaemic cardiomyopathy.
This language outlines a minimum duration of three months of DAPT for certain patients who may need to interrupt or discontinue the medication for a variety of reasons and supports the strong safety profile for Promus Element and Promus Element Plus stents.
A semi-structured survey suggests that more than 15% of patients suffering from severe symptomatic aortic stenosis eligible for transcatheter aortic valve implantation (TAVI) did not receive the treatment for budget-related reasons in 2011.
After three years of follow-up, the benefits of TAVI were sustained as measured by all-cause mortality, cardiovascular mortality, repeat hospitalisation, and functional status, according to new data presented at TCT.
The ISAR-LEFT MAIN 2 trial is the first and largest performed randomised, multicentre comparison trial between zotarolimus-eluting and everolimus-eluting stents in unprotected left main coronary artery lesions.
"This analysis adds provocative new insight to ongoing discussions about the minimal duration of dual antiplatelet therapy required to safeguard patients with the latest generation drug-eluting stents," said Sigmund Silber, presenter of the RESOLUTE data at TCT.
Under this FDA-approved IDE, up to 880 ST-elevation myocardial infarction (STEMI) patients at 50 sites in the USA and worldwide will be enrolled in the APPOSITION V clinical trial.
The studies evaluated hospital readmission rates for subsequent heart attack and initial hospitalisation costs in patients with acute coronary syndrome treated with a percutaneous coronary intervention and antiplatelet therapy, including prasugrel or clopidogrel.
The PrimeWire Prestige Plus device will be used in six new Volcano-sponsored clinical studies to explore new indications in stable and unstable coronary artery disease, peripheral arterial disease and neurology.
The Kona Surround Sound system utilises focused ultrasound, delivered from outside the body, to treat the nerves leading to and from the kidney.
Riociguat demonstrates a statistically significant improvement in the six-minute walk test, a predictor of improved outcome in patients suffering from pulmonary arterial hypertension.
The primary endpoint, freedom from all-cause mortality from procedure to 30 days, was met at 97%. Freedom from all cause cardiovascular mortality at 30 days was 100%.
BioMatrix Flex significantly reduced the risk of clinical events compared with Cypher Select, which was associated with a reduced risk of very late stent thrombosis.
The Parachute Ventricular Partitioning Device (CardioKinetix), available now in eight sizes, is a minimally invasive treatment used for percutaneous ventricular restoration therapy.
Permaseal is a transapical access device designed to enable self-sealing, sutureless cardiac access and closure of structural heart repair procedures including TAVI and mitral valve repair.
The Platinum Long Lesion study met its primary endpoint of target lesion failure at 12 months with a 3.2% rate for the Promus Element Stent (Boston Scientific) in the per protocol population compared to a pre-specified performance goal of 19.4% based on historical outcomes for the Taxus Express Stent.
Gore is introducing the technology in the REDUCE study for prevention of recurrent stroke in patent foramen ovale patients.
Medtronic has announced new findings from the Medtronic CoreValve ADVANCE study for one year survival and health-related quality of life.
The Resolute drug-eluting stent from Medtronic delivered successful clinical results two challenging characteristics of coronary artery lesions commonly found in patients with diabetes.
Approved by the FDA under an investigational device exemption, the study will evaluate the safety and effectiveness of the device in approximately 370 patients at up to 30 sites in the USA.
New data presented at the TCT congress show sustained blood pressure reduction and safety with Symplicity Renal Denervation system in patients with treatment-resistant hypertension.
An analysis of the benefits to using a fractional flow reserve-guided intervention strategy found that the technology can improve patient outcomes while saving significant amounts of money.
Acute cardiac care experts who met at Acute Cardiac Care Congress 2012 in Istanbul, Turkey, said that patients with severe chest pain should call the emergency number immediately as pre-hospital care is essential to survival.
Data suggest Sidegaurd stent has long term benefits in treating bifurcation disease
The expanded indication includes patients with aortic valve stenosis who are eligible for surgery, but who are at high risk for serious surgical complications or death.
Denise Fischer, Homburg, Germany, says that renal denervation may have a positive effect in psychological processes, stress perception and processing as well as in quality of life.
A patient with a functional bicuspid valve is less likely to require repeat valvuloplasty, aortic valve replacement, death, or a heart transplant than a patient with other types of valve morphology, according to a study published by Shiraz Maskatia, Texas Children's Hospital, Houston, USA, and colleagues in Catheterization and Cardiovascular Interventions.
Akshay Bagai, Toronto, Canada, and colleagues have found that mentored simulation may help less proficient cardiac catheterisation operators to improve their skills.
The US prospective, multicentre, single-arm, clinical study will evaluate the safety and efficacy of the new Gore Septal Occluder in the treatment of percutaneous, trancatheter closure of ostium secundum atrial septal defect.
Cibiem will develop a proprietary, minimally invasive, catheter-based approach focused on carotid body modulation for the treatment of sympathetic nervous system-mediated diseases.
In the survey, conducted by the Society for Cardiovascular Angiography and Interventions (SCAI), four out of five patients say their lives have changed for the better following angioplasty.
The device, which is also CE-marked, features a balloon specifically designed to prevent balloon ruptures in transcatheter aortic valve implantation (TAVI) and balloon aortic valvuloplasty (BAV) procedures.
The report published online in The Journal of the American College of Cardiology (JACC) analyses data from 1.1 million patients who underwent diagnostic cardiac catheterisation procedures and 941,248 patients treated with percutaneous coronary intervention from January 2010 to June 2011.
The collaboration is focused on integrating Infraredx's true vessel characterisation (TVC) Imaging System with Philips' Allura Xper catheterisation (cath) lab imaging systems
Data from the FAME II trial has shown that patients with fractional flow reserve (FFR)-guided stenting plus the best available medical therapy had superior outcomes to those treated with medical therapy alone.
APPOSITION III is a prospective, single-arm, multicentre, post-market trial to assess the long term performance of the Stentys Self-Apposing stent in routine clinical practice in 1,000 STEMI patients.
CircuLite has announced that its Synergy Circulatory Support System will be included in four presentations at the 24th Annual Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium (Miami, USA, 22–26 October).
The four day training course offered hands-on training, analysis of cardiac CT data sets including post-percutaneous coronary intervention and surgery assessment skills.
Darby, who has 20 years of experience in the medical device industry, with most of those in the interventional cardiovascular space, was the business unit head of Bayer Interventional before joining Svelte.
The company specialises in the development of non-invasive technology for advanced haemodynamic monitoring, which can be used in the surgical, intensive care, emergency room and cardiology settings.
The company has been commercialising its CE-marked paclitaxel-coated drug-coated balloon for coronary (Primus) and peripheral (Legflow) applications since March 2012.
The results of the clinical trial with the Boston Scientific repositionable and retrievable device are expected to be used to support CE mark and other international regulatory approvals.
The study with the Revivent Myocardial Anchoring System for use in Less Invasive Ventricular Enhancement procedures commenced in Europe under the guidance of Louis Labrousse, Bordeaux-Pessac, France.
A scientific presentation and a live procedure of adjustment of the MiCardia device were presented by Martin Andreas and Markus Czesla to 200 cardiac surgeons and other professionals in Stuttgart, Germany.
The system gives healthcare professionals 24-hour access to digital inventory management, communities, eLearning, product information and technical support in order to reduce costs.
Results from the Argentine Registry of Acute Coronary Syndromes (SCAR) were presented by Ricardo Villarreal at the 38th Argentine Congress of Cardiology in Buenos Aires.
Under the agreement, Terumo will continue to incorporate BioMatrix in the production, marketing and selling of its drug"eluting stents in markets worldwide, outside of the United States.
The new study, published in the International Journal of Epidemiology, also found that sudden death was associated independently with poor educational attainment.
The study in Brisbane, Australia, is the first in which researchers examined the association between daily average temperature and "years of life lost" due to cardiovascular disease.
TRV027 has shown improvement in haemodynamics and has been well-tolerated in patients with stable advanced heart failure, supporting advancement into a phase 2b study in acute decompensated heart failure patients.
The draft quality standard on hypertension describes measurable markers of high-quality, cost-effective care to drive improvements in the effectiveness, safety and experience of care for people with hypertension.
The MediGuide Technology reduces the need for fluoroscopic images during cardiovascular procedures.
According to a study published in the European Journal of Heart Failure, severe aortic stenosis patients with low-mean gradient and reduced left ventricular ejection fraction (LVEF) have worse outcomes after transcatheter aortic valve implantation (TAVI) than patients with preserved LVEF and high-mean gradient.
Athanase Benetos, Nancy, France, and colleagues have found that higher blood pressure levels in very old patients do not predict mortality or major cardiovascular events but low pulse pressure amplification in this population does predict such events.
According to the company, the next-generation system will feature a new four electrode catheter that delivers radiofrequency energy simultaneously and is designed to significantly reduce ablation time during renal denervation procedures.
The announcement was made at a press conference in London by Boris Johnson, Mayor of London; Kevin Murphy, CEO of ExCeL London and Kim Fox, representing the ESC.
A team of researchers at the Erasmus University Medical Center, Rotterdam, The Netherlands, performed a cost-analysis of 84 propensity matched surgical and transcatheter aortic valve implantation patients. Ruben Osnabrugge, one of the authors of the study, writes for Cardiovascular News key conclusions of the analysis and compares it with the economic outcomes reported from the PARTNER trial.
New data presented at the PCR London Valves 2012 meeting has shown a 97% procedural success implantation with the CoreValve System (Medtronic) through a direct aortic approach.
The clinical trial is an open-label study that will randomise subjects 1:1 to renal denervation vs. no denervation with both groups receiving maximal tolerated doses of antihypertensive medications.
The CE-marked Carillon Mitral Contour System is designed to reduce functional mitral regurgitation while reducing mitral annulus dilatation upon device deployment.
ASSURE will evaluate the ability of RVX-208, Resverlogix's BET protein inhibitor, to regress atherosclerotic disease versus placebo using intravascular ultrasound (IVUS) technology in patients with coronary arterial disease.
The Emerge Catheter (Boston Scientific) is a next-generation pre-dilatation balloon catheter designed to treat challenging lesions in coronary arteries.
The new catheter to implant the Stentys Self-Apposing Stent adds a hydrophilic (slippery) coating and an ergonomic handle that considerably facilitates stent implantation in tortuous vessels.