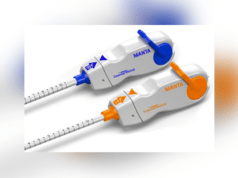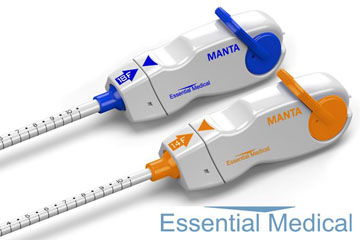
Essential Medical has announced the completion of the 60-day follow up for its MANTA US pivotal study. Manta is vascular closure device and is designed to close punctures ranging from 10Fr to 25Fr at femoral arterial access sites after percutaneous cardiac procedures that use a large-bore device, such as transcatheter aortic valve implantation (TAVI) and ventricular assist devices.
Closure of large bore femoral access sites has been associated with significant morbidity including long times to achieve haemostasis, extended procedure time, need for a vascular surgeon in the catheterisation lab, delayed ambulation, higher rate of complications and higher total cost of care. Therefore, the aim with Manta is to address the complexities of closing these large punctures in high-pressure vessels using novel closure technology. It is intended to provide reliable and repeatable deployment with immediate haemostasis in order to reduce complications associated with large bore closure. At present, the Manta has the CE mark but is an investigational device in the USA and Canada.
Gary Roubin, chief medical officer of the company, states: “We are adding to the growing pool of data with the longer term 60-day follow up for the MANTA device. The data from this study of 341 patients along with our over 3,000 commercial cases in parts of Europe continues to provide us with great confidence in the clinical benefits of the Manta device. Our team is on track to submit our PMA to the FDA in the next 90 days as we continue to make great progress towards enabling safer and more effective large bore closure in the USA.”