Tag: Manta
Key learnings from Manta device study to be presented at TCT
Teleflex has announced that Magnus Settergren, associate professor at the Karolinska Institutet, int...
First patients enrolled in Manta Ultra vascular closure device study
Teleflex has announced the first patient enrolment in a clinical study that is intended to demon...
US FDA grant premarket approval to Manta vascular closure device
Teleflex has received premarket approval (PMA) from the US FDA for the Manta vascular closure de...
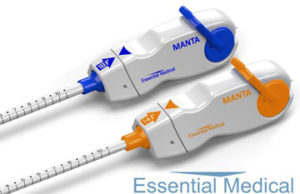
Teleflex buys vascular closure company Essential Medical
Teleflex has acquired Essential Medical, which has developed and commercialised the CE marked Ma...
TVT 2018: Positive results for Manta large bore closure device
Results from the SAFE MANTA IDE clinical trial—the first pivotal trial for a dedicated large bor...
Sixty-day follow-up for the MANTA US pivotal trial completed
Essential Medical has announced the completion of the 60-day follow up for its MANTA US pivotal ...
Essential Medical completes enrolment in US IDE trial of Manta
Essential Medical has announced the completion of enrolment in the US pivotal investigational device...