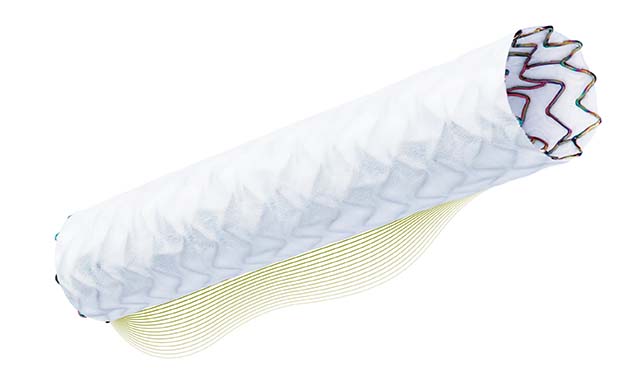
The US FDA has approved the PK Papyrus covered coronary stent system (Biotronik) under Humanitarian Device Exemption for use in the emergency treatment of acute coronary perforations. A press release reports that the PK Papyrus is the first FDA approved device for the treatment of acute perforations of native coronary arteries and coronary bypass grafts in vessels 2.5mm to 5mm in diameter, adding that the device received the CE mark in 2013.
According to the press release, the covered stent is available in 17 sizes, expanding treatment options and helping avoid the need for emergency coronary artery bypass grafting. PK Papyrus is the only 5Fr compatible covered coronary stent available in the USA.
The ultrathin, single-stent design and electrospun polyurethane membrane contribute to PK Papyrus’ superior performance. The device is 58% more flexible and has a 23% smaller crossing profile compared to Graftmaster. The latter has a layered dual-stent design and is the only other covered coronary stent available in the USA.
Dean Kereiake (The Christ Hospital and Vascular Center, Cincinnati, USA), says: “In rare cases of a coronary perforation, time is the enemy. The device’s superior flexibility and tracking means that PK Papyrus delivers more like a conventional stent and can treat a perforation more quickly, avoiding further complications. Hospitals need to have this potentially lifesaving device ready for physicians to use.”
Marlou Janssen, president of Biotronik, states: “Perforation is very uncommon, but physicians need to be fully prepared for this emergency event. It is unacceptable that this critical care area has seen no innovation in nearly two decades. PK Papyrus has been trusted in various international markets since 2013. Biotronik saw a need, rose to the occasion and invested in bringing this stent to the USA. We made this decision because patient care is paramount and we are committed to helping physicians and hospitals save lives and improve patient outcomes.”













