Tag: Biotronik
Teleflex completes acquisition of Biotronik’s Vascular Intervention business
Teleflex today announced that is has completed the previously announced acquisition of substantially...
Trial of DCB and scaffold technologies enrols first CTO-PCI patients
Biotronik has announced the enrolment of the first patient in the Leave Nothing Behind-trial, which ...
Teleflex announce acquisition of Biotronik’s Vascular Intervention business
Teleflex has today announced it has entered into a definitive agreement to acquire Biotronik’s Vascu...
BIOMAG-I two year results confirm safety and efficacy of Biotronik resorbable magnesium scaffold
New two-year follow-up data from the BIOMAG-I first-in-human trial confirms a reliable and predi...
First patient enrolled in BIOMAG-II trial of Freesolve resorbable scaffold
Biotronik has announced the enrolment of the first patient in the BIOMAG-II trial aiming to evaluate...
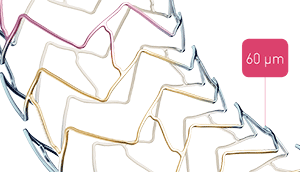
Biotronik’s Freesolve resorbable magnesium scaffold gains CE mark
Biotronik has announced the CE approval and launch of the Freesolve resorbable magnesium scaffold (R...
Study establishes safety and efficacy of ticagrelor monotherapy in acute coronary syndrome patients treated with Orsiro
According to recently presented TICO trial data, a press release reports, patients with acute co...
Orsiro Mission gets CE mark
Biotronik has received CE mark certification for the Orsiro Mission drug-eluting stent (DES) sys...
Biotronik appoint company’s first chief medical officer
David Hayes, former physician administrator at Mayo Clinic, Rochester, USA, has been appointed c...
ESC 2019: Ultrathin strut Orsiro better than thin strut Xience for STEMI patients
One-year data from the BIOSTEMI study, simultaneously published in The Lancet and presented at t...
Biotronik announces positive data for Magmaris
A press release from Biotronik highlights data from the BIOSOLVE-IV registry outlining procedura...
Biotronik launches PK Papyrus covered coronary stent in the USA
Biotronik has launched the PK Papyrus covered coronary stent system in the United States for use...
CRT 2019: Early resorption and low complications demonstrated for Magmaris
A press release reports that preliminary data from the BIOSOLVE-IV registry strengthen the clini...
CRT 2019: Three-year data shows trend towards lower target vessel failure with Orsiro vs. Resolute Integrity and Synergy
Three-year outcome data from the BIO-RESORT randomised controlled trial, which were presented at...
New Australian research and development partnership aims to innovate heart failure care
Biotronik, the University of Newcastle (in Australia), and the John Hunter New England Local Health ...
FDA approves Orsiro for use in the USA
The US FDA have approved Biotronik’s drug-eluting stent Orsiro. A press release states...
Registry data for Biotronik’s bioresorbable scaffold Magmaris supports its roll-out into clinical practice
Preliminary data from the BIOSOLIVE-IV registry are consistent with the favourable safety outcom...
TCT 2018: Orsiro continues to have lower rates of target lesion failure than Xience
Two-year data from the BIOFLOW-V randomised trial—which compared a sirolimus stent with a biodeg...
US FDA approves PK Papyrus stent for coronary perforations
The US FDA has approved the PK Papyrus covered coronary stent system (Biotronik) under Humanitar...
ESC 2018: Higher all-cause mortality with Orsiro at five years than with Xience
According to the five-year results of the BIOSCIENCE trial, sirolimus-eluting stents with a biod...
EuroPCR 2018: One- to two-year landmark analysis shows significantly lower target lesion failure with Orsiro vs. Resolute Integrity
Two-year outcome data from the BIO-RESORT randomised controlled trial, which were presented in a...
EuroPCR 2018: Further evidence for safety and efficacy of Magmaris bioresorbable scaffold
Data presented during EuroPCR (22 May – 25 May, Paris, France) provide further evidence on its c...
CIT 2018: BIOFLOW-VI confirms non-inferiority of Orsiro to Xience
Nine and 12-month data for Orsiro—presented by Yang Yue Jin (Fu-Wai Hospital, National Center of...
Japanese Ministry of Health approve Biotronik’s Orsiro drug-eluting stent
According to a Biotronik press release, the Japanese Ministry of Health has approved the company...
TCT 2017: Pooled analysis of Magmaris studies continue to show no instances of scaffold thrombosis at 12 months
Data presented during the 2017 Transcatheter Cardiovascular Therapeutics (TCT) meeting (29 Octob...
Enrolment in BIOVITESSE trial of polymer-free coronary stent initiated
Biotronik has announced the start of enrolment in a coronary stent trial aiming at assessing the saf...
ESC 2017: Significantly lower event rates with Orsiro than with Xience
Results from the BIOFLOW V study indicate that patients who undergo percutaneous coron...
EuroPCR 2017: Low risk of scaffold thrombosis with Magmaris
Data presented at EuroPCR (16–19 May, Paris, France) indicate that a metallic bioresorbable scaf...
Magnesium 1,000 programme reveals high deployment success for Biotronik’s Magmaris scaffold
Biotronik has released details of the clinical performance of its Magmaris magnesium scaffold in...
BIOFLOW-IV confirms non-inferiority of Biotronik’s Orsiro in Japan
The 12-month results of Biotronik’s BIOFLOW-IV multicentre clinical study have been presented at...
FDA approves Biotronik’s Pro-Kinetic Energy bare metal stent
Biotronik’s Pro-Kinetic Energy bare metal stent has received FDA approval, which is based on the...
TCT 2016: Physicians discuss their positive experiences of using a metallic scaffold
At a symposium at the 2016 Transcatheter Cardiovascular Therapeutics (TCT) meeting (29 October-2...