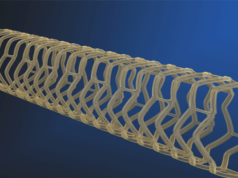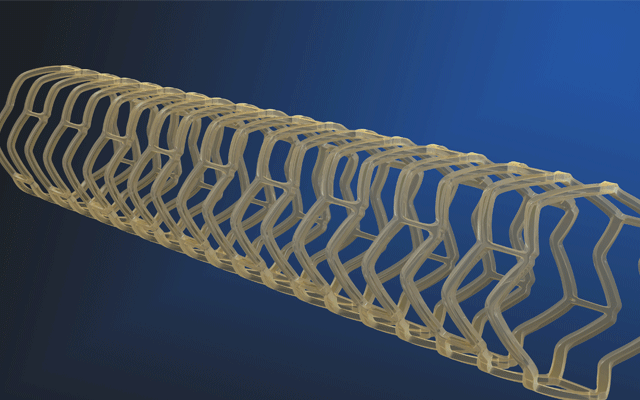
The Fantom bioresorbable scaffold (Reva Medical) shows continued positive clinical outcomes according to an interim data set from the FANTOM II clinical trial, which was presented at the 2017 Transcatheter Cardiovascular Therapeutics (TCT) meeting this week. These interim data were for 125 patients followed through 24 months and indicated a low rate of major adverse cardiac event (MACE) of 5.6% and a single very late scaffold thrombosis event.
According to a press release, Reva Medical previously reported a MACE rate of 4.2% through 12 months for the complete 240-patient data set with a single scaffold thrombosis event in the subacute time frame. In addition to the latest clinical follow up, a 25-patient subset in the trial underwent angiographic imaging to determine late lumen loss at 24 months. This showed a final in-scaffold late loss of 0.25 mm, which is in the desired range of 0.2 mm to 0.4 mm.
The data were presented in a moderated poster session by trial investigator, Ricardo A Costa (Institute Dante Pazzanese of Cardiology, Sao Paulo, Brazil) and in an oral presentation by James B. Hermiller (Heart Center of Indiana, Indianapolis, USA).
Costa comments: “This preliminary data set from the FANTOM II trial provides physicians with an early look at two-year clinical results for the Fantom scaffold. The low MACE rate and in-scaffold late lumen loss measurement are encouraging as they demonstrate sustained safety and performance of Fantom out to 24 months in this group of patients.”
The FANTOM II trial is evaluating the safety and performance of the Fantom sirolimus-eluting bioresorbable coronary scaffold in 240 patients outside of the USA. Six-month data from the FANTOM II trial was used as the basis for CE Marking for the device, which was granted in April of this year.
As well as unveiling these data, Reva Medical has also revealed plans for its next-generation scaffold – Fantom Encore – that, the press release reports, will have a market-leading 95micron strut thickness for the 2.5mm diameter scaffold. It will use the same polymer as Fantom called Tyrocore, the company’s proprietary tyrosine-derived polymer designed specifically for vascular scaffold applications. Tyrocore is inherently radiopaque, making Fantom and Fantom Encore visible under fluoroscopy. Additionally, Tyrocore is strong, enabling thinner struts while retaining radial strength.
Hermiller says: “Reva’s announcement of 95micron strut thickness for Fantom Encore in the 2.5mm diameter is a promising development for bioresorbable scaffold technology. Bioresorbable scaffolds have the potential to offer patients short-term benefits of metallic stents without the long-term complications. Thinner struts have been associated with improved deliverability and vessel healing.”