Jocelyn Hudson
Merit Medical names Martha Aronson as new president and CEO
Merit Medical recently announced the appointment of Martha Aronson as the company's new president an...
Teleflex completes acquisition of Biotronik’s Vascular Intervention business
Teleflex today announced that is has completed the previously announced acquisition of substantially...
Endospan announces positive 30-day outcomes from TRIOMPHE IDE study
Endospan recently announced the presentation of 30-day results from the statistical dissection p...
Inquis Medical’s Aventus thrombectomy system found safe and effective for patients with PE
Inquis Medical recently announced results from its AVENTUS trial evaluating the safety and efficacy ...
Evident Vascular raises Series B financing to advance AI-powered IVUS platform technology
Evident Vascular has announced the successful closing of its Series B financing with new investors S...
AVS completes US$36 million Series B financing ahead of IVL US commercial launch
Amplitude Vascular Systems (AVS) recently announced that it has completed a Series B round of financ...
TCT 2024: Drug-eluting resorbable scaffold proves cost effective at one year in LIFE-BTK analysis
A retrospective economic analysis of the LIFE-BTK trial has demonstrated the one-year cost-effec...
CMS grants transitional pass-through payment for Medtronic’s Symplicity Spyral and Recor’s Paradise renal denervation systems
The Centers for Medicare and Medicaid Services (CMS) has granted transitional pass-through (TPT) pay...
Philips announces US FDA approval for enhanced LumiGuide guidewire, marks 1,000th patient treated with 3D device guidance technology
Royal Philips today announced the introduction of the 160cm US Food and Drug Administration (FDA...
New ESC guidelines combine peripheral arterial and aortic diseases for first time, emphasising interconnectivity of whole arterial system
The European Society of Cardiology (ESC) has today published its 2024 guidelines for the management ...
SCOREPAD seeks to address “alarmingly high” mortality in CLTI patients with underlying coronary disease
A new research letter underscores the need to improve long-term survival following lower extremi...
SCOREPAD seeks to address “alarmingly high” mortality in CLTI patients with underlying coronary disease
A new research letter underscores the need to improve long-term survival following lower extremi...
“There’s no meeting like it”: Stage is set for CX Aortic Live 2024
A meeting centred around live case transmissions from across the world, CX Aortic Live 2024 (7–8...
US FDA approves Cordis’ Mynx Control venous vascular closure device
Cordis recently announced that the US Food and Drug Administration (FDA) has granted premarket appro...
Johnson & Johnson completes acquisition of Shockwave Medical
Johnson & Johnson today announced it has completed its acquisition of Shockwave Medical. Shockwa...
Endospan releases early TRIOMPHE IDE study results at STS 2024
Endospan shared 30-day results from the first 22 patients enrolled in the TRIOMPHE investigation...
Simona Zannetti returns to Medtronic to lead aortic business
Medtronic recently announced that Simona Zannetti has been appointed as the general manager of i...
Cook Medical announces Slip-Cath Beacon Tip catheter is now available in the USA and Canada
Cook Medical has announced that its Slip-Cath Beacon Tip hydrophilic selective catheter is now a...
Inari Medical announces first patient enrolment in PEERLESS II randomised controlled trial
Inari Medical has announced the first patient enrolment in PEERLESS II. This prospective, global, ...
REAL-PE demonstrates statistically significant lower major bleeding rates with Ekos system compared to mechanical thrombectomy for PE treatment
Data from the REAL-PE study, presented at TCT 2023 (23–26 October, San Francisco, USA) demonstra...
TCT 2023: LIFE-BTK breathes life into drug-eluting resorbable scaffolds in breakthrough for below-the-knee arteries
Results of the LIFE-BTK randomised controlled trial have just been presented at TCT 2023 (23–26 ...
Obituary: Roger M Greenhalgh 6th February 1941 – 6th October 2023
Roger Malcolm Greenhalgh, the surgeon internationally renowned for his unparalleled contribution...
FastWave Medical secures multi-million dollar private financing within weeks
FastWave Medical recently announced the swift closure of an oversubscribed multi-million dolla...
RapidAI announces US$75 million growth investment led by Vista Credit Partners
RapidAI today announced US$75 million in Series C funding led by Vista Credit Partners, a subsidiary...
Boston Scientific announces position on FDA update about use of paclitaxel-coated devices to treat PAD
Following yesterday's news that the US Food and Drug Administration (FDA) has changed its stance on ...
Artificial intelligence could enable life-saving early diagnosis and advance the treatment of pulmonary hypertension
Thirona recently announced its new artificial intelligence (AI)-based algorithm for pulmonary artery...
Bentley launches its first product in the USA
Bentley has announced the US launch of its BeBack crossing catheter, which is designed for the treat...
Akura Medical announces successful first-in-human use of its mechanical thrombectomy platform
Akura Medical announced today it has initiated its first-in-human clinical study of the Akura mechan...
CX 2023: Statins save lives after aortic repair regardless of dose
Statin treatment after aortic repair is associated with improved long-term survival, while dose ...
BASIL-2 points towards endovascular-first revascularisation strategy in CLTI patients
A question from Manj Gohel (Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK) on w...
AVS’s pulsatile IVL technology attracts an additional US$8.8 million to close US$28.8 million Series B round
AVS, an early-stage medical device company focused on safely and effectively treating severely c...
Vivasure Medical announces FDA IDE approval to initiate US pivotal study
Vivasure Medical has announced that the US Food and Drug Administration (FDA) has granted an investi...
New VOYAGER PAD analysis confirms consistent benefit of low-dose rivaroxaban plus aspirin following lower extremity revascularisation
Data from a new prespecified analysis of the phase III VOYAGER PAD clinical trial show that low-...
Results on ReCor Medical’s Paradise ultrasound renal denervation system published in two JAMA Network publications
ReCor Medical and its parent company, Otsuka Medical Devices, recently announced that primary en...
Viz.ai launches AI-powered Viz Vascular Suite
Viz.ai has announced the launch of Viz Vascular Suite—artificial intelligence (AI)-powered softw...
Aortic advances: New techniques and technologies in the spotlight
Heinz Jakob (Essen, Germany) and Tilo Kölbel (Hamburg, Germany) offer their respective cardiac a...
Mortality drops for acute type A aortic dissection, still high for patients not receiving surgery
The chance of a patient surviving after an acute type A aortic dissection has improved sig...
FLASH results demonstrate “excellent safety profile” of the FlowTriever system in full US cohort
Results of the FLASH registry demonstrate the “excellent safety profile” of the FlowTriever syst...
Medtronic announces co-promotion agreement with CathWorks, with path toward acquisition
Medtronic today announced it has entered into a strategic partnership agreement with CathWorks, a pr...
SoniVie receives FDA IDE approval for pilot study to treat hypertension with renal artery denervation TIVUS technology
SoniVie recently announced that on 5 May 2022 the US Food and Drug Administration (FDA) granted inve...
Shockwave Medical and Genesis MedTech obtain regulatory approval in China for intravascular lithotripsy
Shockwave Medical and Genesis MedTech Group announced today that they have successfully obtained app...
Vivasure Medical announces Series D financing to advance portfolio of PerQseal vessel closure devices
Vivasure Medical has announced the closing of the first tranche of €22 million (US$23 million) as pa...
Artificial intelligence could make endovascular aortic repair outcomes more predictable
At the 2022 Charing Cross (CX) International Symposium (26–28 April, London, UK), Tom Carrell (C...
CX returns to in-person format once more in the London spring
Charing Cross (CX) chair Roger Greenhalgh welcomes the vascular community to this year's symposium, ...
Death due to cardiovascular disease more likely among Black adults born in the USA
Black adults born in the USA had a higher rate of death from cardiovascular diseases and all cau...
Study suggests a more proximal landing zone is preferred for TEVAR of acute type B aortic dissections
A review evaluating the results of thoracic endovascular aortic repair (TEVAR) following acute t...
New publication “extends evidence for clinical benefit” with CytoSorbents’ haemoadsorption technology into type A dissection aortic surgery
CytoSorbents has called attention to a new publication which reports on the use of intraoperative ha...
Prolonged TV watching may increase risk of venous thromboembolism
A new study reports that watching TV for four hours a day or more is associated with a 35% higher ri...
Philips integrates cloud-based AI and 3D mapping into its mobile C-arm system series
Royal Philips today announced physicians will now have access to advanced new 3D image guidance ...
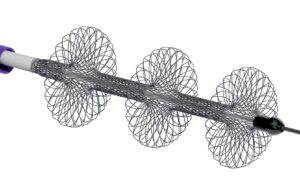
Cardiovascular Systems announces development of IVL technology for the treatment of coronary and peripheral arterial diseases
Cardiovascular Systems recently announced that it has made significant progress towards the commerci...
Vesalio initiates clinical study evaluating enVast thrombectomy system in patients with STEMI
Vesalio recently announced the start of enrolment in its NATURE study using enVast, the company’s fi...
Machine learning may help identify people at risk of thoracic aortic aneurysm
A team led by investigators at Massachusetts General Hospital (MGH) in Boston, USA, recently used de...
Research reveals improved outcomes and decreased mortality in management of aortic dissections with care plans designed by multidisciplinary expertise
The Minneapolis Heart Institute Foundation (MHIF; Minneapolis, USA) has announced the publication of...
Type A dissection enters a new revolution
“We are at the beginning of a type A dissection revolution,” Joseph E Bavaria (University of Pen...
New technologies will simplify and shorten aortic surgical procedures while reducing radiation
In a session on “Reduction of radiation challenges” at CX Aortic Vienna 2021 (5–7 October, broad...
Patients should get a choice between open and endovascular surgery for aortic conditions, CX audience agrees
A decisive vote at CX Aortic Vienna 2021 (5–7 October, broadcast) revealed that 90% of the audie...
Open surgery remains a well-accepted as option to treat challenging aortic arch
Results of a poll conducted at CX Aortic Vienna 2021 (5–7 October, broadcast) indicated that ope...
First patient treated with Cardiovascular Systems’ ViperCross peripheral support catheter
Cardiovascular Systems recently announced that the first patient has been successfully treated with ...
MedAlliance announces first patient enrolment in SELUTION DeNovo study
MedAlliance today announced first patient enrolment in the coronary randomised controlled study SELU...
EuroPCR 2021: Late-breaking data demonstrate long-term benefits of Medtronic radiofrequency renal denervation in real-world hypertensive patients
Medtronic today announced new clinical data from the Global SYMPLICITY registry (GSR) indicating...
Results from EXTRACT-PE trial of Penumbra’s Indigo aspiration system published
Results from the EXTRACT-PE trial, which found that Penumbra’s Indigo aspiration system met its ...
Inaugural CX Aortic Vienna to take place online
The inaugural CX Aortic Vienna meeting will be livestreamed 8–11 September 2020 to an international,...
Philips and the US government collaborate in ventilator production ramp up to combat COVID-19 pandemic
Royal Philips has announced that the US government and Philips agreed to team up to increase the pro...
ACS defends healthcare workers as PPE shortages lead to acrimony
The American College of Surgeons (ACS) today leapt to the defense of healthcare workers who are ...
Virtual ACC: Clopidogrel exposure along with aspirin and rivaroxaban should be “minimised or avoided” in PAD revascularisation
“More bleeding with background clopidogrel, even if not severe by adjudication, may be associate...
Virtual ACC: Antiplatelet drugs increase risk for TAVI patients with atrial fibrillation
In the POPULAR TAVI trial, patients with atrial fibrillation who took oral anticoagulants alone ...
New guideline: ASA no longer recommended to prevent first stroke, heart disease
A new Heart & Stroke guideline published today in the Canadian Medical Association Journal does ...
ACURATE neo helps facilitate early discharge following TAVI procedures
NOTE: This video is ONLY available to watch in selected countries and geographies
Rajesh ...
Ablative Solutions publishes data from the Peregrine post-market study
Ablative Solutions has revealed that positive six-month results from the Peregrine post-market s...
Medtronic begins pilot study as part of SPYRAL HTN clinical programme for renal denervation in hypertension patients
Medtronic has announced that it will begin enrolment in a pilot study evaluating the safety and effi...
IVL has “changed the dynamics” of facilitating EVAR & TEVAR in calcified iliac arteries
Frank Arko (Charlotte, USA) talks to VEITHtv at the VEITHsymposium 2019 (19–23 November, New Yo...
Shockwave IVL can aid in eliminating recoil and dissection during below-the-knee angioplasty
George Adams (Raleigh, USA) talks to VEITHtv at the VEITHsymposium 2019 (19–23 November, New Yor...
ECHSA 2019: Shape matters and is an innovation
Martin Kostelka (Leipzig, Germany), who was the first to use the CardioCel 3D (Admedus) product ...
CardioCel benefits patients with different anatomies for intracardiac application
Speaking to Cardiovascular News at the ECHSA 2019 meeting (European Congenital Heart Surgeons As...
CardioCel 500—Long term experience with no calcification
Giovanni Stellin (Padova, Italy) talks to Cardiovascular News at the ECHSA 2019 meeting (Europea...
Renal denervation still holds strong potential as a therapy for hypertension
Although renal denervation may not hit the heights originally envisaged for it in the management of ...
Low-dose polypill could be used for primary prevention of cardiovascular events
A polypill that combines four cardiovascular drugs has been shown to reduce the risk of major cardio...
FARXIGA meets primary endpoint in Phase III DAPA-HF trial
AstraZeneca has announced positive results from the Phase III DAPA-HF trial which showed that FA...
CeloNova appoints new president and CEO
CeloNova has announced the appointment of Carl J St. Bernard as president and chief executive office...
FDA approves new device to improve symptoms in patients with advanced heart failure
The US Food and Drug Administration has approved the Barostim Neo system for the improvement of ...
Novel wireless pressure monitoring system will have an “enormous impact” on HF patient care
Horst Sievert (Frankfurt, Germany) talks to BLearning at EuroPCR 2019 (21–24 May, Paris, France)...
Announcing the TCT 2019 late-breaking trials and science
The Cardiovascular Research Foundation (CRF) has announced the 12 late-breaking trials and 16 late-b...
Cardiovascular Systems acquires Gardia Medical’s Wirion embolic protection system
Cardiovascular Systems recently announced that it has acquired the Wirion embolic protection sys...
Transcatheter repair of tricuspid valves appears safe and effective when surgery is not an option
Data from two new studies suggest that transcatheter tricuspid valve repair (TTVr) is a safe and...
Hormone therapy linked to heart fat and hard arteries
Hormone replacement therapy is a common treatment for menopause-related symptoms, and new research f...
SeQuent Please ReX drug-coated PTCA balloon catheter receives FDA breakthrough device designation
B Braun Interventional Systems has announced that the US Food and Drug Administration (FDA) has gran...
EuroPCR 2019: Allegra Transcatheter Heart Valve shows “very good haemodynamic outcomes”
Ulrich Schäfer (Hamburg, Germany) speaks to Cardiovascular News at EuroPCR (21–24 May, Paris) ab...
TRILUMINATE trial shows “extremely favourable” results in patients with tricuspid regurgitation
Speaking to Cardiovascular News at EuroPCR 2019 (21–24 May, Paris, France), Georg Nickenig (Bonn...
ADVERTORIAL: Edwards PASCAL™ Transcatheter Valve Repair System is innovative
This content is only for readers outside of the US as it discusses a device that is not FDA appr...
Philips launches IntraSight interventional applications platform
Royal Philips recently announced the launch of the new IntraSight interventional applications pl...
Boston Scientific initiates trial comparing left atrial appendage closure to direct oral anticoagulants for stroke risk reduction post-AFib ablation
According to a press release, Boston Scientific has initiated the OPTION trial to compare safety...
Medtronic launches Telescope guide extension catheter
Medtronic recently announced the global launch of the Telescope guide extension catheter, a newl...
CE mark for Watchman FLX left atrial appendage closure device
Boston Scientific announced it has received CE mark and initiated a limited market release of the ne...
Vascular Dynamics names Martin Rothman chief medical officer
Martin T Rothman has been appointed as chief medical officer of Vascular Dynamics, effective 1 Janua...
Vascular Dynamics names Martin Rothman chief medical officer
Martin T Rothman has been appointed as chief medical officer of Vascular Dynamics, effective 1 Janua...
Philips launces Azurion with FlexArm to aid image-guided procedures
Philips launches its Azurion with FlexArm to aid patient imaging and positioning during image-gu...
Positive results for LivaNova’s Perceval sutureless valve
Three newly published studies highlight the safety, efficacy, and economic advantage of the Perv...
Abbott to acquire Cephea Valve Technologies
It was recently announced that Abbott has exercised its option to purchase Cephea Valve Technologies...
New JACC publication reinforces valve of a coronary CTA + HeartFlow Analysis-guided pathway for diagnosing heart disease
HeartFlow has announced the publication of the PACIFIC substudy showing that their FFRct Analysi...
JenaValve Technology appoints John T Kilcoyne as chief executive officer
Medical device executive John T Kilcoyne has been appointed as chief executive officer of JenaValve ...
Shockwave initiates US pivotal study for coronary intravascular lithotripsy
Shockwave Medical has initiated its US Food and Drug Administration (FDA) Investigatio...
First patients in the Middle East treated with Diamondback 360 Coronary Orbital Atherectomy System
The first patients in the United Arab Emirates (UAE) have been treated with the Diamon...
Sleeping less than six hours a night may increase cardiovascular risk
People who sleep less than six hours a night may be at increased risk of cardiovascular disease comp...
FDA approves world’s first device for treatment of premature babies and newborns with an opening in their hearts
The US Food and Drug Administration (FDA) has approved the Amplatzer Piccolo Occluder (Abbott), ...
Boston Scientific, Edwards Lifesciences agree to global litigation settlement
Boston Scientific and Edwards Lifesciences today announced that the companies have reached an agreem...
Does your insurance card matter when you have a heart attack?
Medicaid reimbursement to healthcare facilities on ST-elevation myocardial management—or STEMI, a se...
SCCT releases new guideline for use of computed tomography in TAVR procedure
The Society of Cardiovascular Computed Tomography (SCCT) has released a new expert consensus doc...
Patient recruitment reaches half-way point for global DAPT study
December marked a significant milestone for a major study into the use of short duration dual an...
First United States treatment with J-Valve TAVI device
The first US patient has been successfully treated with JC Medical's transfemoral transcatheter ...
Sapien 3 Ultra receives FDA approval
It has been announced that the Sapien 3 Ultra system (Edwards Lifesciences) has received US FDA ...
Internationally renowned cardiac surgeon joins University of Miami Health System
Joseph Lamelas, an internationally recognised expert cardiac surgeon who helped pioneer minimall...
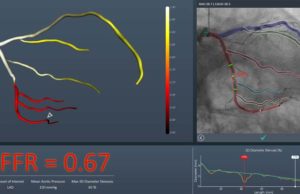
FFRangio system receives US FDA clearance
US FDA 510(k) clearance has been granted for the FFRangio System (CathWorks). The FFRangio syste...
Boston Scientific exercises option to acquire Millipede
Boston Scientific has exercised its option to acquire the remaining shares of Millipede, upon it...
First patients treated in United States with OrbusNeich Teleport Microcatheter
The first patients in the United States have been treated using the OrbusNeich Teleport Microcathete...