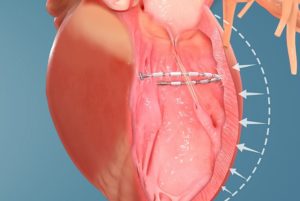
Ancora Heart has been given US Food and Drug Administration (FDA) approval of its investigational device exemption (IDE) application for the CorCinch-HF pivotal study, which is designed to evaluate the safety and efficacy of the AccuCinch Ventricular Repair System in patients with heart failure and reduced ejection fraction (HFrEF).
A company statement says the AccuCinch System is the first completely percutaneous device designed to directly reshape the left ventricle of the heart, thereby addressing the fundamental issue in the progression of systolic heart failure.
The CorCinch-HF pivotal study is a prospective, randomised, open-label, multicentre, international, clinical safety and efficacy investigation of the AccuCinch System, which is designed to enrol 400 patients at up to 80 centres worldwide. The study design is for an initial analysis of safety and clinical efficacy for PMA submission after the first 250 patients have reached six months of follow-up, and then a second analysis after the entire cohort has reached 12 months of follow-up. The study will ultimately follow patients through five years post treatment to track long-term results.
“The approval of the IDE for the CorCinch HF pivotal trial represents a major milestone as we continue to gather data to evaluate the safety and effectiveness of the AccuCinch System,” states Jeff Closs, President and CEO of Ancora Heart in the press release. “We look forward to working with study sites to initiate patient enrolment as soon as possible.”












