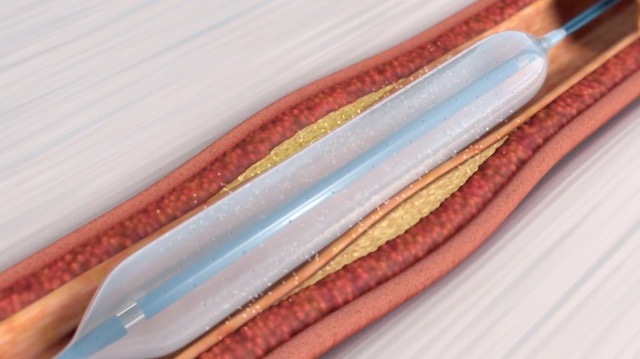
The Virtue (Orchestra BioMed) sirolimus-eluting balloon for the treatment of coronary in-stent restenosis has secured breakthrough device designation from the US Food and Drug Administration (FDA).
Breakthrough designation for a medical device or a device-led combination of products enables manufacturers to expedite the development, assessment, and review of a product and allows for frequent interactions and feedback from the FDA during the premarket review phase. In a press release, Orchestra BioMed says that through the program it can expect prioritised review of its submission for Virtue SEB.
It describes it as the first and only non-coated sirolimus-eluting angioplasty balloon system to show promising clinical results, and says it has the potential to offer significant advantages for treatment of coronary in-stent restenosis.
In the press release, Darren R. Sherman, president, chief operating officer and founder of Orchestra BioMed, said: “We believe Virtue SEB addresses an important unmet clinical need and provides an improved treatment alternative for a patient population with limited options. It is the first and only non-coated angioplasty balloon that provides arterial delivery of sirolimus, the proven gold standard drug used on drug-eluting stents for preventing restenosis of treated arteries. We plan to fully leverage the benefits of FDA Breakthrough Device designation as we seek to accelerate the U.S. clinical and regulatory development of Virtue SEB with the goal of providing physicians and patients with the benefits of our novel therapeutic device.”












