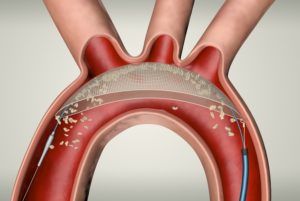
European CE mark has been awarded to the TriGUARD 3 cerebral embolic protection (CEP) device from Keystone Heart, a Venus Medtech Company.
According to the company, TriGUARD 3 is the only CE marked product designed to cover and protect all three major cerebral aortic arch vessels. It consists of a Nitinol frame and dome-shaped mesh deflector that is delivered transfemorally and designed to “self-position” in the aortic arch. This design, says Keystone Heart, allows the TriGUARD 3 CEP device to conform to a variety of patient anatomies.
Chris Richardson, Keystone Heart president and chief executive officer, says: “Taking into consideration the devastating impact of stroke, we are pleased to bring this important technology to patients undergoing any transcatheter heart procedure. The introduction of the TriGUARD 3 CEP device in Europe provides physicians the only commercially available device that is designed to protect all three cerebral vessels.”
The device is designed to minimise the risk of cerebral damage by deflecting embolic debris away from cerebral circulation during transcatheter aortic valve implantation (TAVI) and other transcatheter heart procedures. Keystone Heart recently completed the REFLECT trial, a pivotal randomised study of the TriGUARD 3 CEP device, and says it is currently finalising data analysis ahead of a planned marketing application to the US Food and Drug Administration (FDA). Keystone Heart is a medical device company that develops and manufactures devices for structural heart interventions.













