 The REFLECT II randomised clinical trial evaluating the safety and efficacy of a cerebral embolic protection device—TriGuard 3 (Keystone Heart)—in patients undergoing transcatheter aortic valve implantation (TAVI), found that the device met its primary safety endpoint compared to historical controls but did not demonstrate superiority for the primary hierarchical efficacy endpoint.
The REFLECT II randomised clinical trial evaluating the safety and efficacy of a cerebral embolic protection device—TriGuard 3 (Keystone Heart)—in patients undergoing transcatheter aortic valve implantation (TAVI), found that the device met its primary safety endpoint compared to historical controls but did not demonstrate superiority for the primary hierarchical efficacy endpoint.
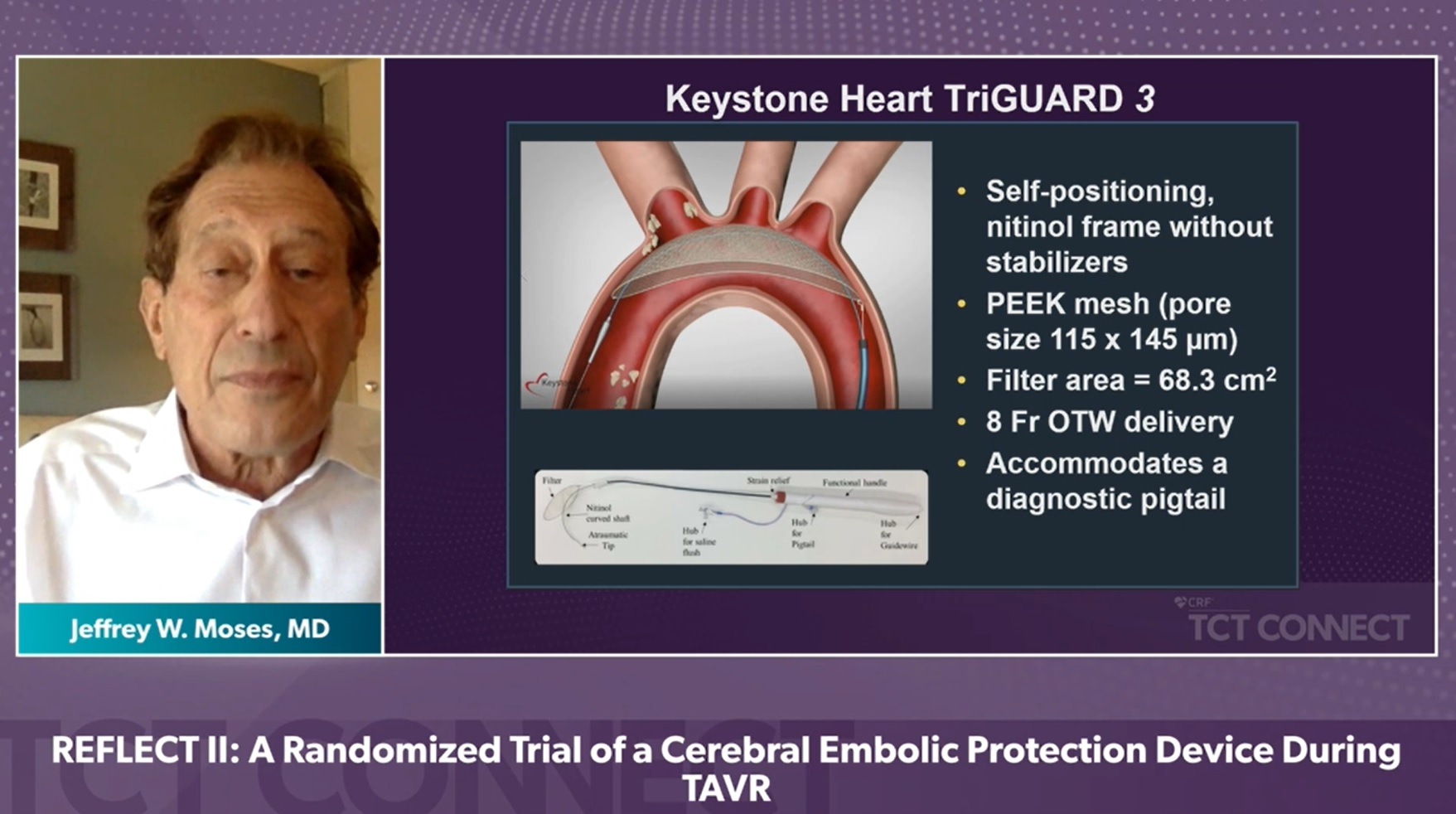
Results of the clinical trial were presented during a late breaking trial session at TCT Connect 2020 (14–18 October, virtual) by Jeffrey W Moses (NewYork-Presbyterian/Columbia University Irving Medical Center, New York, USA). The REFLECT II trial evaluated the safety and effectiveness of the TriGuard 3 (TG3), a self-stabilising cerebral embolic deflection filter, in patients undergoing TAVI. The study intended to randomise 295 patients 2:1 to TAVI with TG3 vs. control.
The primary safety endpoint was a composite of all-cause mortality, stroke, life-threatening or disabling bleeding, stage 2/3 acute kidney injury, coronary artery obstruction requiring intervention, major vascular complication, and valve-related dysfunction requiring intervention (VARC 2 defined) at 30 days. The endpoint was compared with a Performance Goal (PG) of 34.4%.
The study’s primary efficacy endpoint was a hierarchical composite of all-cause mortality or stroke at 30 days, National Institute of Health Stroke Scale (NIHSS) worsening, absence of diffusion-weighted magnetic resonance imaging (DWI) lesions post-procedure, and total volume of cerebral lesions (TLV) by DWI. Cumulative scores derived by the Finkelstein-Schoenfeld method were summed for each patient and compared between groups.
The REFLECT II analysis population included 283 patients: 41 roll-in, 121 randomised to TG3 and 121 controls—with 58 randomised in phase II and 63 pooled from REFLECT phase I. TG3 was delivered and positioned in the aortic arch prior to TAVI in 100% of cases and retrieved intact in all cases.
After enrolment of 179 of the 225 planned randomised patients, the sponsor suspended trial enrolment. After limited unblinding and review of the data, Keystone Heart decided to formally close the study and proceed with a marketing application.
Outlining the results at TCT, Moses reported that TG3 met the primary safety endpoint (22.5% vs 34.4% PG, p non-inferiority=0.0001). However, superiority for the primary efficacy endpoint was not met, with similar win-ratios and win percentage (TG3 0.84 (45.7%) vs 1.19 (54.3%), p=0.857) between groups. Median TLV was not different with TG3 protection (215.39mm3 vs 188.09mm3, p=0.405).
“Compared to controls, the primary 30-day safety endpoint was higher with TriGuard 3 due primarily to TAVI-related vascular and bleeding complications,” said Moses. “While the study did not demonstrate superiority of TriGuard 3 compared to pooled controls for the primary hierarchical efficacy endpoint, a post hoc DW-MRI analysis suggests that TG3 may reduce larger ischaemic lesions. Improved device stability to achieve reliable, complete cerebral coverage may improve outcomes.”











