Tag: US FDA
US FDA announces plans to address medical device shortage risks
The US Food and Drug Administration (FDA) has issued a statement outlining measures to enhance p...
US FDA issues first draft guidance for AI-based medical device development
The US Food and Drug Administration (FDA) has issued draft guidance that includes recommendations to...
SoniVie announces advancements in its renal denervation programme
SoniVie, which has developed a novel Therapeutic Intra-Vascular Ultrasound System (TIVUS) to treat a...
US FDA approves Cordis’ Mynx Control venous vascular closure device
Cordis recently announced that the US Food and Drug Administration (FDA) has granted premarket appro...
CX 2024: Paclitaxel device restrictions “did cause harm”
“Unfortunately, we are doing worse for our patients today,” were the sobering words of Eric Sece...
TriClip gets positive vote from US FDA advisory panel
The Circulatory System Devices Panel of the Medical Devices Advisory Committee for the US Food and D...
TCT 2023: First presentation of complete patient-level dataset on paclitaxel and death that helped sway the US FDA
Data from a patient-level meta-analysis—a factor in the US Food and Drug Administration’s (FDA) ...
Timeline: Key milestones in the paclitaxel story
The release of a letter from the US Food and Drug Administration (FDA) concerning the use of paclita...
Long-awaited US FDA update finds data do not support excess mortality risk for paclitaxel-coated devices
In a letter to healthcare providers dated 11 July 2023, the US Food and Drug Administration (FDA...
Swiss parliament votes to accept US FDA-approved medical devices
The Swiss Federal Assembly has voted in favour of accepting medical devices with US Food and Drug Ad...
FDA issues two final guidances for including patient perspectives in medical device studies
The US Food and Drug Administration (FDA) has issued two final guidances providing recommendations f...
First coronary patient enrolled in FDA IDE study of drug eluting balloon
MedAlliance has announced enrollment of the first patient in its study of Selution SLR 014” drug...
Ancora Heart receives IDE approval for study of AccuCinch
Ancora Heart has been given US Food and Drug Administration (FDA) approval of its investigationa...
FDA issues IDE for Impella ECP and EUA for Impella RP
Abiomed has received investigational device exemption (IDE) from the US Food and Drug Administra...
FDA issues new policy on remote monitoring devices for use during COVID-19 pandemic
The US Food and Drug Administration (FDA) has issued an immediately-in-effect guidance that allo...
FDA issues update on reducing the risk of patient infections during cardiac surgery
The US Food and Drug Administration (FDA) has cleared a new version of the LivaNova Heater-Cooler Sy...
“Our goal is to treat more patients with mitral regurgitation”
Last month, Abbott announced US Food and Drug Administration (FDA) approval of a new trial—REPAI...
US FDA grants Breakthrough Device designation to CorFlow Infusion System
CorFlow Therapeutics has announced that the company has been granted Breakthrough Device designa...
SeQuent Please ReX drug-coated PTCA balloon catheter receives FDA breakthrough device designation
B Braun Interventional Systems has announced that the US Food and Drug Administration (FDA) has gran...
Scott Gottlieb resigns as head of US FDA
The commissioner of the US Food and Drug Administration (FDA), Scott Gottlieb, has unexpectedly ...
US government shutdown disrupting FDA work
The US government shutdown means the country’s Food and Drug Administration (FDA) cannot accept ...
First United States treatment with J-Valve TAVI device
The first US patient has been successfully treated with JC Medical's transfemoral transcatheter ...
Sapien 3 Ultra receives FDA approval
It has been announced that the Sapien 3 Ultra system (Edwards Lifesciences) has received US FDA ...
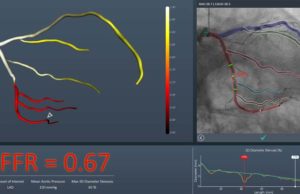
FFRangio system receives US FDA clearance
US FDA 510(k) clearance has been granted for the FFRangio System (CathWorks). The FFRangio syste...
First patients treated in United States with OrbusNeich Teleport Microcatheter
The first patients in the United States have been treated using the OrbusNeich Teleport Microcathete...
Europe braces for harder times in medical device innovation while US FDA eases regulations
Countries in the European Union have long been the first to receive new innovations in medical techn...
US FDA agrees to 1000-patient pivotal trial of Armetheon’s tecarfarin
Armetheon has reached agreement with the US Food and Drug Administration (FDA) for a single 1000-pat...
US FDA grants Fast Track designation to Angionetics’ Generx coronary heart disease gene therapy
The US Food and Drug Administration (FDA) has granted Angionetics “Fast Track” designation for the p...
Robert Califf to resign as US FDA commissioner
Robert Califf is to step down as US Food and Drug Administration (FDA) commissioner upon the ina...
US FDA bans powdered gloves
The US Food and Drug Administration (FDA) has issued a final rule to ban powdered surgical glove...
NICE collaborates with US FDA on Payer Communication Taskforce
The UK’s National Institute for Health and Care Excellence (NICE) has announced its participatio...