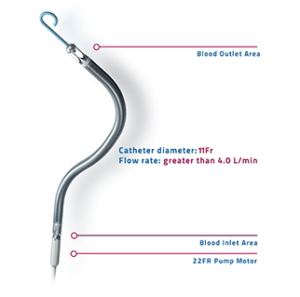
Abiomed has received investigational device exemption (IDE) from the US Food and Drug Administration (FDA) for an early feasibility study with a first-in-human trial of Impella ECP (expandable cardiac power), a 9 French (Fr) heart pump. It will be studied in high-risk percutaneous coronary intervention (PCI) patients. The FDA has also issued an emergency use authorisation (EUA) for Impella RP to include patients suffering from COVID-19 related right heart failure or decompensation, including pulmonary embolism (PE). Impella RP is a temporary heart pump that provides circulatory support for patients who develop right side ventricular failure.
The primary endpoint of the prospective, multicentre, non-randomised early feasibility study of Impella ECP is successful delivery, initiation and maintenance of adequate haemodynamic support and a composite rate of major device-related adverse events during high-risk PCI. The study protocol will enrol and treat up to five US patients who require revascularisation. If successful, enrolment will be expanded to additional patients, pending approval from the FDA. Patient enrolment is expected to begin later this calendar year for the first-in-human trial.
Impella ECP is the world’s smallest heart pump. It is available for investigational use only and is not approved for use outside of clinical studies.
The Impella RP is FDA approved to treat right heart failure or decompensation following left ventricular assist device implantation (LVAD), myocardial infarction, heart transplant, and open-heart surgery; it is CE marked to treat right heart failure or decompensation following LVAD, myocardial infarction, heart transplant, open-heart surgery, or refractory ventricular arrhythmia.
In its authorisation letter, the FDA writes, “Based on extrapolation of data from the approved indication and reported clinical experience, FDA has concluded that the Impella RP may be effective at providing temporary right ventricular support for the treatment of acute right heart failure or decompensation caused by COVID-19 complications, including PE.”
The EUA authorises the use of the Impella RP by healthcare providers (HCP) in the hospital setting for providing temporary right ventricular support for up to 14 days in critical care patients with a body surface area ≥1.5m2, for the treatment of acute right heart failure or decompensation caused by complications related to Coronavirus Disease 2019 (COVID-19), including PE.










