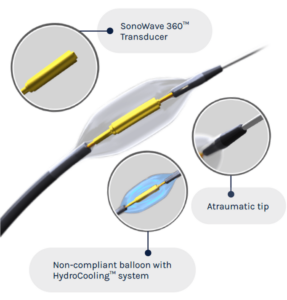
ReCor Medical and its parent company, Otsuka Medical Devices, announced consistent and significant blood pressure-lowering results across a range of patients with uncontrolled hypertension, including across differences in age, sex, baseline blood pressure, medication level and ethnicity. The results come from analysis of the pooled data from ReCor’s RADIANCE global clinical trial programme: three prospectively powered, randomised and sham-controlled clinical trials which evaluated the endovascular Paradise ultrasound renal denervation (uRDN) system in patients with uncontrolled hypertension.
The results were presented at the 2022 American Heart Association (AHA) annual meeting (5–7 November, Chicago, USA) by Ajay Kirtane, professor of medicine at Columbia University and an interventional cardiologist at New York-Presbyterian/Columbia University Irving Medical Center (both New York, USA).
The RADIANCE pooled analysis includes data from more than 500 patients randomised in the three studies from ReCor’s RADIANCE global programme: RADIANCE-HTN TRIO, which studied patients with resistant hypertension, and RADIANCE-HTN SOLO and RADIANCE II, which studied patients with mild-moderate hypertension. The combined dataset showed an overall reduction in daytime ambulatory systolic blood pressure in the uRDN group of -8.5mmHg (p<0.0001) with a difference between treatment and sham at two months of -5.9mmHg (p<0.0001), favouring uRDN. Blood pressure results were similarly positive in the 24-hour, nighttime, home, and office measures. A favourable safety profile was consistently observed following uRDN treatment across the studies.
“Pooling the data from the RADIANCE program demonstrates that treatment with the Paradise uRDN System results in a consistent reduction in blood pressure across differing severities of hypertension. The consistent and clinically meaningful blood pressure reduction across multiple patient groups increases our interest in the use of uRDN as a potential therapeutic option, when added to lifestyle modification and medications for our patients with uncontrolled blood pressure,” said Kirtane, study co-principal investigator.
“It is very important that the RADIANCE pooled analysis demonstrated a consistent blood pressure reduction in patients across a range of hypertension and both with and without antihypertensive medication, thus broadening the potential applicability of uRDN. Just as important, more than 50% of patients treated with uRDN in the pooled analysis either achieved daytime ambulatory blood pressure control or had a greater-than-10mmHg decrease in daytime ambulatory systolic blood pressure at two months, showing the potential benefits of uRDN as an element of a treatment regimen for patients with uncontrolled hypertension,” said study principal investigator Michel Azizi, professor of medicine at Université Paris Cité, Hôpital Européen Georges Pompidou, Paris, France.
The RADIANCE global programme is an international, multicentre initiative designed to explore the benefits of ultrasound renal denervation in hypertension. The RADIANCE studies are double-blind, randomised, sham-controlled trials designed to provide information about the ability of the Paradise uRDN System to treat high blood pressure. All three studies in the RADIANCE global programme were individually powered for efficacy with a primary endpoint of daytime systolic ambulatory blood pressure at two months, and all three met their primary efficacy endpoint with statistical significance at two months.
If maintained in the long-term, the blood pressure reductions demonstrated in the RADIANCE pooled analysis correlate to a potential 25% reduction in cardiovascular risk as shown in a meta-analysis of the cardiovascular benefits of antihypertensive medications.