Two-year outcomes from the MITRAL trial, presented at PCR Valves e-course (22-24 November, virtual) suggest that transeptal mitral valve-in-valve replacement should be considered as the standard of care for high risk patients with a favourable anatomy.
This was the conclusion of Mayra Guerrero (Mayo Clinic Hospital, Rochester, USA) who shared data from the study evaluating transcatheter valve-in-mitral annulus calcification (MAC), valve-in-ring and valve-in-valve approaches, which were studied through the MITRAL trial.
“We know that transcatheter mitral valve replacement is becoming an alternative for high surgical risk patients with MAC or failed surgical bioprosthesis and prior repairs with surgical rings,” Guerrero said in her presentation introduction adding, however, that there is limited data available on these procedures. This prompted the need for the MITRAL trial, she explained, which is the first prospective trial to evaluate the safety and feasibility of valve-in-mitral annulus MAC, valve-in-ring and valve-in-valve procedures.
Presenting data from the study, Guerrero noted that the valve-in-valve procedure had a very low mortality rate (6.7%, n=2), compared to 43.3% and 39.3% in the valve-in-ring and MAC groups respectively. “I don’t think this is a surprise as these are very different patient populations,” explained Guerrero, with lower ejection fraction at baseline in the valve-in-ring group, as well as difference in the procedures.
In the MAC arm, half of the patients were treated with transatrial and half with transeptal access, based upon anatomy, Guerrero explained, and she commented that a lower mortality rate was observed at 30 days for the transeptal group (6.7% vs. 21.4%). However, she commented that there was no statistical difference due to the small sample size, and that the difference was no longer present at two years.
Overall mortality at two years in the entire cohort for the MAC arm was 39%, she noted, “essentially the same, or perhaps numerically lower than the two year mortality with TAVR in the Partner I trial – so I think this is highly encouraging,” Guerrero commented.
Strokes were recorded in between 3.3-10.7% of patients across the three groups after two years, more haemolytic anaemia in the valve-in-ring and MAC arms, which led to a higher reintervention rates in these two patient groups.
Discussing the mean mitral valve gradients, Guerrero said that gradients remained stable at two years in all of the groups, but tended to be higher in patients treated with a smaller size, and the difference was statistically significant in the valve-in-ring group.
There was a sustained improvement of symptoms, she added, with most of the patients in NYHA class III-IV at baseline, and most of them remaining class I-II at two years.
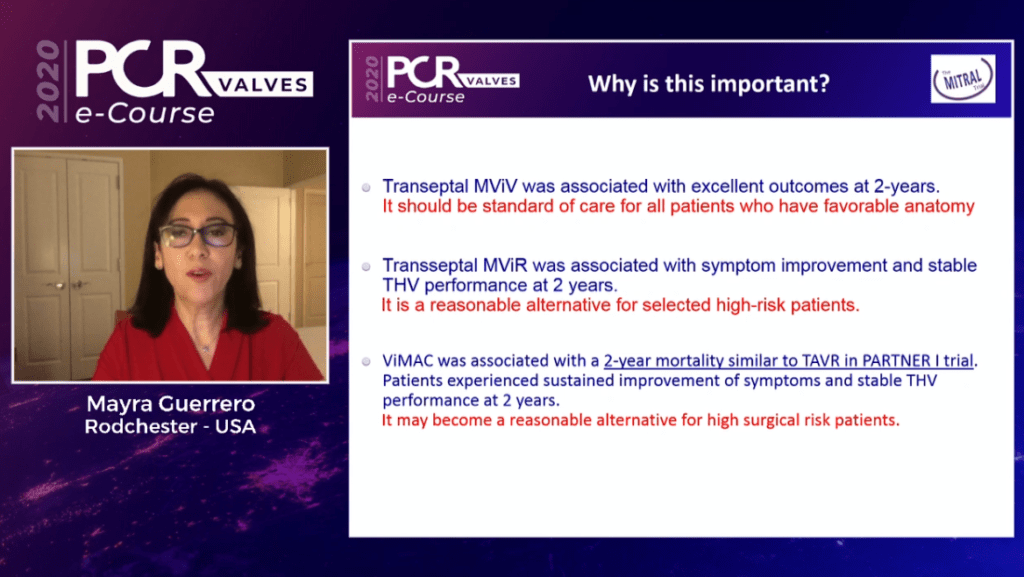
In her concluding remarks, Guerrero commented that transeptal mitral valve-in-valve was associated with excellent outcomes at two years, and should be considered as the standard of care for all patients who have favourable anatomy. Transeptal mitral valve-in-ring was associated with symptom improvement and stable transcatheter heart valve performance at two years, prompting Guerrero to comment that it is a reasonable alternative for selected high surgical risk patients. Finally, she commented that valve-in-MAC was associated with a two-year mortality similar to TAVI as seen in the PARTNER I trial, commenting that it may become a reasonable alternative for high surgical risk patients.
Further study will be carried out through the MITRAL 2 clinical trial, she added.













