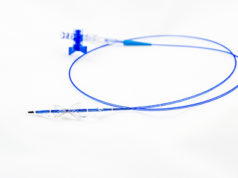
Neovasc has received approval of the US FDA to initiate the COSIRA-II IDE clinical trial. The trial’s purpose will be to demonstrate the safety and effectiveness of the Reducer system for treatment of patients with refractory angina. Once completed, the trial data is intended to support an application to the FDA for approval to begin marketing Reducer in the USA.
COSIRA-II will be a 380 patient, multicentre, randomised (1:1 ratio), double blinded, sham-controlled clinical trial with up to 35 investigational centres across North America. The design of COSIRA-II is very similar to that of the COSIRA study—a 104-patient study previously conducted in Europe and Canada. The positive results of that study were published in The New England Journal of Medicine, February 2015.
Neovasc CEO Alexei Marko comments: “Since its commercial launch in Europe two years ago, Reducer has consistently provided relief of severe symptoms in patients suffering from refractory angina, resulting in significant improvements in their quality of life. We are eager to replicate our European clinical and commercial success in the United States, by introducing this important new therapy for patients who have no other option for managing their chronic, severe chest pain.”
Neovasc is currently evaluating start up timelines and funding options for the COSIRA-II trial.