 The Japanese Ministry of Health, Labor and Welfare (MHLW) has approved the recommendation by the Central Social Insurance Medical Council (Chuikyo) to provide reimbursement for HeartFlow FFRct Analysis. The HeartFlow Analysis has already received regulatory approval from the Japanese Pharmaceuticals and Medical Devices Agency (PMDA). Reimbursement will go into effect beginning 1 December 2018, enabling greater access for patients with suspected coronary artery disease.
The Japanese Ministry of Health, Labor and Welfare (MHLW) has approved the recommendation by the Central Social Insurance Medical Council (Chuikyo) to provide reimbursement for HeartFlow FFRct Analysis. The HeartFlow Analysis has already received regulatory approval from the Japanese Pharmaceuticals and Medical Devices Agency (PMDA). Reimbursement will go into effect beginning 1 December 2018, enabling greater access for patients with suspected coronary artery disease.
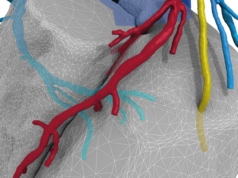
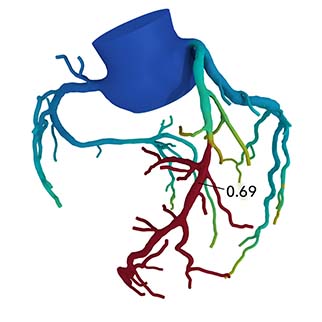
A press release reports that the HeartFlow Analysis is a non-invasive, personalised cardiac test that applies artificial intelligence to image data taken from a standard coronary computed tomography (CT) scan to create a digital 3D model of the patient’s arteries. It then applies advanced algorithms to solve millions of complex equations to assess the impact any blockages have on blood flow to the heart. According to the press release, the HeartFlow Analysis is provided via a secure online interface to offer actionable information to enable clinicians to determine the optimal course of treatment.
The HeartFlow technology has been demonstrated to reduce unnecessary and invasive diagnostic coronary angiography procedures, which can be associated with bleeding, stroke, major blood vessel damage and other serious complications. It also significantly reduces healthcare costs for hospitals.1
Takashi Akasaka (Department of Cardiovascular Medicine, Wakayama Medical University, Wakayama, Japan) says: “When a patient presents with symptoms suggesting coronary artery disease, we want to be able to quickly and effectively diagnose patients while reducing the need for unnecessary tests or invasive procedures. In clinical studies, we were able to see first-hand how the HeartFlow Analysis can help to improve patient management and avoid invasive procedures in some patients. The reimbursement approval will enable more physicians and patients to obtain benefits from this ground-breaking technology.”
John H Stevens, president and chief executive officer, comments: “The reimbursement approval in Japan is an important milestone for HeartFlow as we work to make our state-of-the-art technology available to more patients around the world. Our commercial launch will begin immediately and we look forward to giving clinicians in Japan a new tool to help them confidently diagnose CAD and determine the optimal treatment path for patients.”